Abstract
The inhibition of angiogenesis is a critical element of cancer therapy, as cancer vasculature contributes to tumor expansion. While numerous drugs have proven to be effective at disrupting cancer vasculature, patient survival has not significantly improved as a result of anti-angiogenic drug treatment. Emerging evidence suggests that this is due to a combination of unintended side effects resulting from the application of anti-angiogenic compounds, including angiogenic rebound after treatment and the activation of metastasis in the tumor. There is currently a need to better understand the far-reaching effects of anti-angiogenic drug treatments in the context of cancer. Numerous innovations and discoveries in biomaterials design and tissue engineering techniques are providing investigators with tools to develop physiologically relevant vascular models and gain insights into the holistic impact of drug treatments on tumors. This review examines recent advances in the design of pro-angiogenic biomaterials, specifically in controlling integrin-mediated cell adhesion, growth factor signaling, mechanical properties and oxygen tension, as well as the implementation of pro-angiogenic materials into sophisticated co-culture models of cancer vasculature.
INTRODUCTION
The inhibition of angiogenesis, the growth of new blood vessels from existing vascular networks [1] has been a critical target for cancer therapeutics since Folkman et al. demonstrated that tumor growth was dependent on the presence of vasculature [2, 3]. These studies motivated the discovery of numerous drugs that can disrupt angiogenesis and may consequently deny tumors of growth factors, oxygen, and nutrients required for growth. The first drugs that passed clinical trials disrupted Vascular Endothelial Growth Factor (VEGF) signaling pathways that contribute to angiogenesis, either as antibodies to VEGF such as Bevacizumab [4] or as inhibitors to VEGF Receptor Tyrosine Kinases such as Sunitinib [5]. Although several drug candidates, such as Bevacizumab, Sunitinib, and Sorafenib [6] have received FDA approval for the treatment of specific types of cancers, others failed to improve patient survival [7]. A summary of existing anti-angiogenic therapies, along with limitations, are found on Table 1 (Reviewed in detail elsewhere [8–14]). Ultimately the disruption of cancer vasculature may be important for limiting tumor growth in patients, but it does not fully eliminate tumors in many cases [15, 16]. Important limitations to therapeutics can render drugs ineffective, including the inability of drugs to access the entirety of cancer vasculature due to disturbed flow, and resistance of cancer endothelial cells to drug treatment [10, 11, 13, 17]. Additionally, due to the complexity of the tumor microenvironment, the use of vascular disruption can generate unintended consequences that reverse the progress of treatment or worsen the patient prognoses [18–20].
Table 1.
Summary of existing anti-angiogenic therapeutic strategies and their limitations.
| Therapeutic Strategy | Target | Known Therapeutics | Limitations | References |
|---|---|---|---|---|
| Growth Factor SignalingAMG386 | VEGF Family | Bevacizumab, Sorefenib, Sunitinib, Herceptin | • Imbalanced growth factor signaling in tumors • Requirement for targeting multiple growth factors • Narrow therapeutic window between effectiveness and cytotoxicity in off-target sites • Limited access to inhibitor targets due to compromised blood flow, organization of tumor vasculature |
4–9 11,17 |
| TGFβ Family | Lerdelimumab, Metelimumab | |||
| FGF Family | FP-1039, E-3810, TKI258 | |||
| Angiopoietin | AMG 386 | |||
| EGF | Cetuximab, Panitumumab, Erlotinib | |||
| IGF | NVP-AEW541, NVP-ADW742 | |||
| Calcium Signaling | NFAT Activation | FK506, Anti-SFRP2 antibody | • Limited access to inhibitor targets due to compromised blood flow | 8, 11, 17 |
| Tumor Endothelial Markers | TEM8,SFRP2 | Anti-SFRP2 antibody | • Limited access to inhibitor targets due to compromised blood flow • Impaired genome/epigenetic changes to endothelial cell behavior • Abnormal expression of target molecules inhibits drug penetration to interior of tumor – binding site barrier |
8, 10, 14, 30 |
| Endogenous angiogenesis inhibitors | Angiostatin | Direct application of the inhibitors, or delivery via original ECM molecules including Collagen IV, Collagen XVIII (Endostatin) | • Narrow therapeutic window between effectiveness and cytotoxicity in off-target sites • Limited access to inhibitor targets due to compromised blood flow • Impaired genome/epigenetic changes to endothelial cell behavior |
8,9, 13, 14 |
| Endostatin | ||||
| Thrombospondin-1 | ||||
| Tumstatin | ||||
| Canstatin | ||||
| Arrestin | ||||
| Extracellular Matrix | Matrix Metalloproteinase | MMP9 to release Angiostatin, Endotstatin, Tumstatin | • Narrow therapeutic window between effectiveness and cytotoxicity in off-target sites • Limited access to inhibitor targets due to compromised blood flow, organization of tumor vasculature |
9, 14 |
| integrins αvβ3, αvβ5 | Cilengitide, Vitaxin, S247 | |||
| Focal adhesion kinase | TAE-226, Pfizer-PF-573,228 | |||
| Tumor Activated Fibroblasts | Fibroblast Activation Protein | Sibrotuzumab, val-prolineboronic acid | • Small molecule inhibitors ineffective in certain tumor types | 8 |
An example of these consequences is angiogenic rebound, where cancer vasculature that was previously disassembled by anti-angiogenic treatment rapidly regrows after the cessation of treatment [18, 21–23]. This is the result of multiple contributing mechanisms, including the generation of a local hypoxic environment, increased expression of VEGF, PlGF, FGF2, SDF-1 and PDGF within the tumor [18, 21–23], and putative transformation of tumor cells into endothelial cells [21–25], all of which can result from initial anti-angiogenic treatment. Another example of an unintended effect is the activation of a metastatic switch in tumors, where cells within the tumor change phenotypes to encourage metastasis [20, 26, 27]. While anti-angiogenic treatment focuses on the deactivation of pro-angiogenic signaling pathways, emerging evidence suggests that those same pro-angiogenic pathways can inhibit the onset of metastasis through inhibition of tumor cell migration and invasion, growth factor production by cancer-associated fibroblasts, and the endothelial-mesenchymal transformation [20, 26, 27].
A final example of an unintended consequence of anti-angiogenesis treatment is vascular normalization, where prior to complete network disassembly the cancer vasculature restructures itself from a tortuous, leaking, dysfunctional vasculature [11, 12, 17, 28–30] into a well-ordered, patent vasculature [21–23, 29, 31] (Fig. 1). Unlike other side effects that hinder cancer treatment, normalization can potentially be exploited to improve the transport of anti-cancer drugs into the interior of a tumor, and thereby increase the effectiveness of cancer therapy [32, 33]. Currently, the clinical effectiveness of combined anti-angiogenesis and anti-cancer treatments is unclear [34] as the timing window of vascular normalization is limited and unknown, and there are not yet effective biomarkers to detect a normalization event in situ [34]. However, recent studies have demonstrated that treating ovarian and colon carcinomas with a combination of bevacizumab and paclitaxel in mouse models enabled a uniform intratumoral distribution of paclitaxel [35], and magnetic resonance images of tumor blood vessels suggest that normalization by bevacizumab may peak at 24 hours after treatment in human metastatic brain tumors [36].
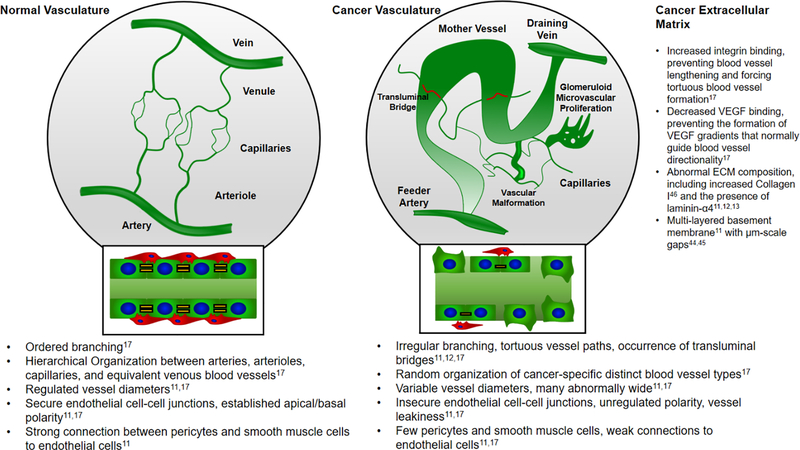
Figure 1.
Normal vasculature and cancer vasculature. Whereas normal vasculature exhibits predictable branching patterns and well-defined arteries, arterioles, capillaries, venules and veins [17], cancer vasculature exhibits chaotic formation of a wide variety of blood vessels that are leaky, tortuous and poorly perfused [11–13, 17, 28–30]. Examples of cancer-specific blood vessels include: Mother Vessels – large, tortuous, leaky vessels; Vascular Malformations – Poorly perfused, abnormally large vessels coated with smooth muscle cells; Glomeruloid Microvascular Prolierations – disorganized, hyperproliferative and hyperperfused vessels; Transluminal Bridges – capillary vessels that penetrate and travel through larger blood vessels; Feeder arteries and Draining veins – tortuous, abnormally large vessels larger than vascular malformations [17].
Importantly, the occurrence of unintended side effects would be difficult to predict via existing angiogenesis assays used for drug discovery in vitro. Most functional assays of anti-angiogenic drug activity only demonstrate specific endpoints of vascular network disruption, such as decreased cell viability, proliferation, migration and/or vascular network formation [37–39]. While these in-vitro assays can be well-suited for discovering compounds that modulate angiogenesis [40], far-reaching effects beyond initial inhibition were not observed in vitro, and largely became known only after they were first observed in animal models [18, 41, 42] and human clinical trials [18, 20, 29, 43].
Advances in the design of biomaterials and cell culture platforms are necessary to enable rapid, detailed characterization of the complex effects of anti-angiogenesis treatments beyond the scope of initial vascular disruption. For example, models of angiogenic rebound will likely require culture environments that support long-term maintenance of vascular networks as well as re-assembly of disrupted vascular networks after the cessation of drug exposure. Models of vascular normalization will require environments that initially promote the formation of tortuous, pathological vasculature so that signs of normalization may be clearly detected. Cell culture systems should additionally accommodate heterotypic co-cultures including tumor-associated cell types to more closely mimic tumor environments. This review will examine recently developed biomaterials and cell culture tools that enable environmental control in vascular morphogenesis models, and may more effectively model anti-angiogenesis treatments.
BIOMATERIALS AS TOOLS TO MODEL VASCULAR MORPHOGENESIS
Angiogenesis consists of a complex series of cell actions, including soluble growth factor signaling, proliferation, migration, and assembly of multi-cellular tubular structures [1], all of which are modulated by the extracellular matrix (ECM) (Fig. 2). In physiological scenarios, microvasculature is in contact with a continuous ECM in the form of a basement membrane composed of Fibronectin, Collagen I, Collagen IV, multiple forms of Laminin, Perlecan and other proteoglycans [44, 45]. In dysfunctional vasculature the ECM contains μm-scale gaps in the basement membrane [44, 45] and elevated levels of ECM proteins, notably Collagen I, leading to increased mechanical stiffness [46]. Additionally, the typical tumor vasculature exists in hypoxic environments that impact behavior of not only endothelial cells but also tumor cells that can modulate angiogenesis via paracrine signaling [18]. Biomaterials are being developed by several groups to recapitulate important cellular and molecular components of pro-angiogenic environments. The following subsections will review biomaterials that present instructive environmental cues that are critical to modulating angiogenesis, including presentation of integrin-binding cell adhesion ligands, mechanical signaling, growth factor signaling, hypoxia and proximity to cancer cell types.
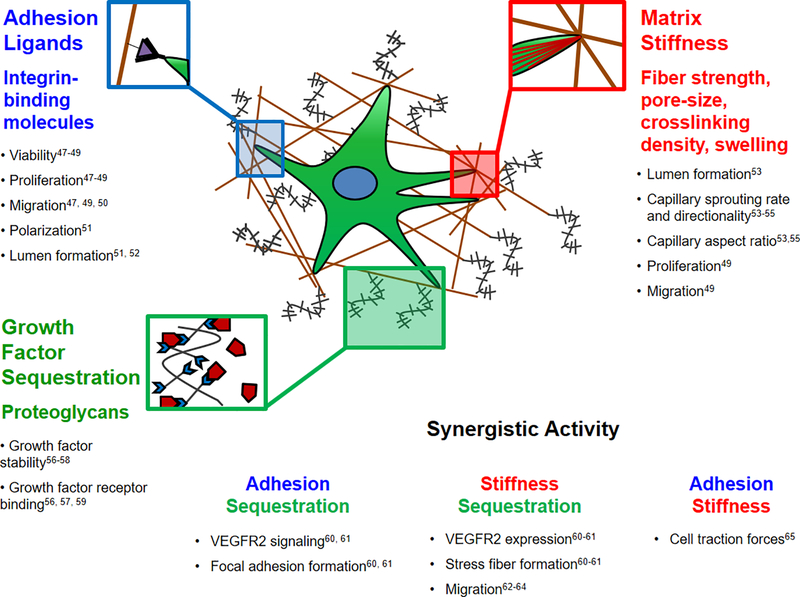
Figure 2.
Individual and synergistic effects of ECM properties on endothelial cell behaviors related to angiogenesis. Integrin-mediated cell adhesion influences endothelial cell viability, proliferation [47–49], migration [47, 49, 50], spatial polarization [51] and lumen formation [51, 52]. ECM modulus influences lumen formation [53], capillary sprouting rate and directionality [53–55], capillary aspect ratio [53, 55], proliferation, and migration [49]. Growth factor sequestration influences growth factor stability [56–58] and facilitates binding between growth factors and receptors [56, 57, 59]. Synergistically, integrin binding and growth factor sequestration influence VEGFR2 activity and focal adhesion assembly [60, 61]. Matrix modulus and growth factor sequestration influence VEGFR2 activity, cytoskeletal stress fiber formation and cell migration rate [62–64]. Finally, integrin binding and matrix modulus influence cell traction forces exerted and detected by endothelial cells [65].
Material Selection
Biologically-derived extracellular matrix components are commonly adapted as biomaterials to induce angiogenesis and endothelial network formation in endothelial cell culture. Collagen, Fibrin and Matrigel can present a wealth of signals that drive pro-angiogenic behaviors of endothelial cells. The often-cited limitations of naturally-derived materials are poor mechanical integrity, high batch-to-batch variation and complex compositions that mask specific mechanisms driving angiogenesis [66, 67]. Although there is difficulty in attributing changes in cell behavior to specific changes in a natural material due to its inherent complexity, studies using natural materials may be coupled to parallel studies on synthetic materials. Comparisons between natural and synthetic materials can reveal new mechanisms that affect angiogenesis, such as the existence of cryptic signaling moieties and growth factor binding sites that are critical to facilitating angiogenesis.
Synthetic, chemically defined biomaterials have been adapted as alternatives to naturally-derived ECM components to achieve greater control of cell behavior in angiogenesis models. Synthetic cell culture materials often consist of background materials that present chemically-defined biological cues to control cell behavior with consistency and predictability, while minimizing the presence of unwanted cues [68–70]. A commonly used material is poly(ethylene glycol) (PEG), a hydrophilic polymer that resists non-specific protein adsorption and presents specific biological signals to cells engineered into the material by chemical modification [71]. Similar materials include poly(vinyl) alcohol (PVA) [72–74], polyacrylamide (pAm) [74], alginate [75], and dextran [76, 77]. A number of studies have modified these materials with biomolecules that control cell behavior through mechanisms including adhesion, growth factor binding and matrix degradation, and these cues may synergistically induce desired angiogenic phenotypes from endothelial cells.
Adhesion Molecules
Among the most critical biomolecules included into a biomaterial are ligands to promote cell adhesion. A commonly used mediator of cell-material adhesion is the integrin-binding Arg-Gly-Asp amino acid sequence (RGD) derived from numerous cell-adhesive proteins present in the ECM [78]. Even though its cell adhesive capabilities are well known and characterized in cell culture systems [79], RGD presentation has been continuously improved in the form of optimized concentrations and spatial patterns to understand and control angiogenesis.
Recent studies have cultured endothelial cells in environments presenting ranges of RGD concentrations to find optimal pro-angiogenic conditions. On the surfaces of PEG hydrogels, optimal RGD concentrations are necessary to encourage endothelial network formation by human umbilical vein endothelial cells (HUVECs). Insufficient RGD density results in cell detachment and excessive RGD results in confluent cell sheet formation [80]. The role of RGD density in modulating pro-angiogenic activity may be defined by the average distance between RGD molecules on a cell culture material. Previous data suggest that this spacing modulates VEGF signaling and cell migration by influencing focal adhesion assembly and focal adhesion kinase (FAK) signaling, and a distance of approximately 44 nm between RGD molecules maximized FAK signaling in bovine aortic endothelial cells [81]. Three dimensional (3D) cultures of HUVECs and aortic ring explants have demonstrated that insufficient concentrations of RGD decrease cell viability, while excessive RGD concentrations result in either arrest of cell migration or excessive matrix degradation through integrin-mediated expression of matrix metalloproteinases (MMPs) [49, 82]. However, a separate study demonstrated that high concentrations of RGD permitted vacuole formation and subsequent lumen formation by endothelial colony forming cells (ECFCs), suggesting the assembly of maturing, patent blood vessels [83]. Taken together, these results reveal that RGD concentrations may encourage angiogenesis or blood vessel maturation, and that appropriate RGD concentrations should be optimized to encourage desired angiogenic outcomes.
Spatial patterning of RGD via photolithography also modulates endothelial tubule formation. One application of spatial patterning is to define where the formation of microvasculature is permitted in a material, but patterning can also impact morphologies and stability of capillary vessels. In one study, the aspect ratios of RGD patterns presented on PEG hydrogels determined whether endothelial cells formed cell sheets or tubule-like structures [84], and 100–200 μm-wide RGD patterns on PEG hydrogels forced HUVECs to form elongated cell aggregates that resembled tubules [85].
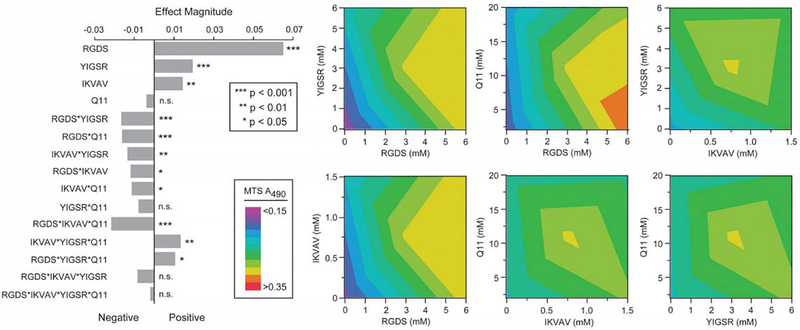
In addition to RGD, other studies have explored the ability of the laminin-mimicking peptides Tyr-Ile-Gly-Ser-Arg (YIGSR) and Ile-Lys-Val-Ala-Val (IKVAV) to modulate pro-angiogenic endothelial cell behaviors. YIGSR or IKVAV presented along with RGD in PEG hydrogels have induced greater tubule formation by HUVECs than with RGD alone [86]. An innovative study from Collier and coworkers explored pro-angiogenic effects in a self-assembly matrix comprised of the acetylated Gln-Gln-Lys-Phe-Gln-Phe-Gln-Phe-Glu-Gln-Gln (QQKFQFQFEQQ) peptide known as “Q11”, which forms β-sheet fibers. These peptides were terminated with cell-adhesive amino acid sequences including Arg-Gly-Asp-Ser (RGDS), Arg-Gly-Asp-Val (REDV), IKVAV and YIGSR [87–89]. The modified Q11 peptides modulated cell adhesion using multiple ligands that were orthogonally added to hydrogel formulations (Fig. 3), and endothelial cell proliferation, viability and adhesion were maximized through optimizing concentrations of multiple species of adhesion ligands [89]. This study demonstrated how systematic control over material properties, coupled with design of experiments methodology can identify important regulators of EC behavior.
Figure 3.
Multifactorial optimization and effects analysis of endothelial cell growth using Q11 modular peptides and cell adhesion ligands [89]. Copyright 2011, with permission from the Royal Society of Chemistry.
While peptides are effective for modulating cell-material adhesion and mimicking some of the functions of ECM proteins, studies have also begun to incorporate full-length ECM proteins into synthetic materials. In one study, human aortic endothelial cells (HAECs) were cultured in the presence of either full-length Collagen or a Collagen-mimicking peptide SCL2–2 presented on PEG hydrogels. SCL2–2 is a Collagen mimicking protein presenting mainly Gly-Phe-Pro-Gly-Glu-Arg (GFPGER) as the integrin-binding domain [90]. HAECs adhered to full-length collagen had greater levels of NOS3 protein expression, greater gene expression levels of NOS3, Thrombomodulin and Selectin-E, and proliferation when compared to HAECs adhered to SCL2–2. While full-length ECM proteins present some advantages, they can also present extraneous background signals into otherwise defined cell culture environments and may require greater control of regiospecific chemistries for incorporation into biomaterials without interfering with essential biological epitopes.
Growth Factor Signaling
Growth factor signaling has been integrated into biomaterials as either short growth factor-mimicking peptides or full-length growth factors. Vascular endothelial growth factor (VEGF) signaling provides an illustrative example. A short peptide termed “QK”, that mimicks the receptor-binding regions of VEGF [91], has been used to enhance angiogenesis. QK was coupled along with RGD to PEG hydrogels and increased the length of endothelial tubule structures relative to those reached on hydrogels functionalized with RGD alone [92]. QK may also be attached to other peptide sequences that facilitate non-covalent incorporation into biomaterials. This enables patterning of QK not just by photopatterning, but also by the use of fluid flow and partial dipping of biomaterials into solutions containing QK, as demonstrated when QK was coupled to hydroxyapatite scaffolds via an attached hydroxyapatite binding sequence [93]. When integrated into Collagen hydrogels via a helical Collagen mimicking domain (CMP) the addition of a CMP-QK peptide induced the formation of elongated endothelial cell structures in two-dimensional (2D) cell culture and enhanced capillary sprouting in three dimensional (3D) culture [94, 95]. Triple-helical hybridization has also coupled QK to PEG hydrogels and produced similar pro-angiogenic effects [85].
Full-length VEGF protein has been coupled to cell culture materials to enhance angiogenic cell behavior. One study covalently immobilized VEGF to PEG molecules through a succinimidyl carbonate group and spatially patterned PEG-VEGF molecules into PEG hydrogels via a scanning confocal laser. The resulting VEGF patterns directed endothelial cell network formation in specific areas of the hydrogels [84]. Full-length VEGF has also increased endothelial cell proliferation when attached to electrospun micro-and nano-fibers of poly(ε-caprolactone) via carbodiimide-mediated carboxylic acid coupling reactions [96]. VEGF was also patterned into Collagen sponges in spatially defined areas to permit formation of microvascular networks. This was achieved by extruding Collagen mixed with an aqueous VEGF solution onto a copper plate, freezing, then inserting the frozen Collagen-VEGF stripes into a larger Collagen sponge [97]. These are only a few examples selected from a larger published literature combining biomaterials with VEGF [98–101], reviewed elsewhere.
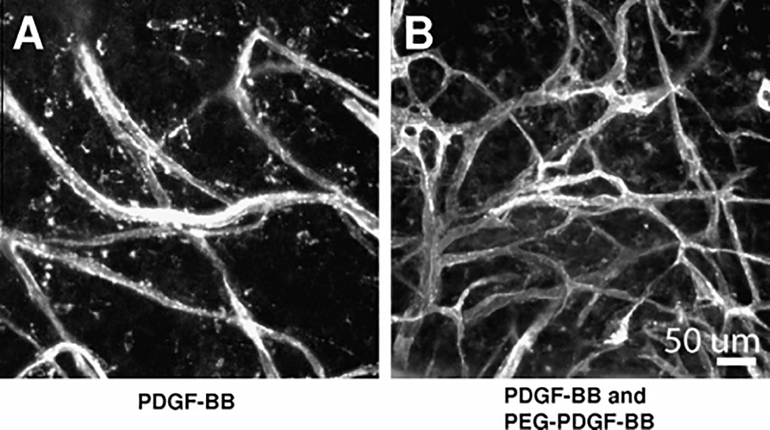
Other growth factors besides VEGF have also been shown to modulate angiogenic cell behaviors when attached to biomaterials. PDGF-BB and bFGF were incorporated into PEG hydrogels via succinimidyl ester coupling to observe effects on HUVEC and pericyte co-cultures. bFGF and PDGF-BB worked in concert to increase tubule network length, albeit by different mechanisms. While PDGF-BB increased pericyte proliferation, bFGF increased HUVEC migration speeds in 2D and 3D cultures. The use of PDGF-BB in endothelial culture systems notably provides the ability to tailor environments to enhance angiogenic activity of perivascular cell types in addition to endothelial cells, and this highlights the need to integrate multiple growth factor signals into pro-angiogenic materials. When PDGF-BB alone was included in hydrogels, endothelial tubule formation occurred whether PDGF-BB was soluble or attached to the hydrogel. When HUVECs were cultured in the presence of releasable PDGF-BB and insoluble Ephrin A1, a matrix-bound receptor tyrosine kinase ligand, the PDGF-BB did not affect endothelial tubule widths in a 2D or 3D environment [102]. Similar hydrogels were implanted in mouse cornea models, but these were modified to contain immobilized PDGF-BB. When exposed to the variety of other cells present in vivo, the presence of covalently immobilized PDGF-BB enhanced the extent of tubule formation (Fig. 4) [103].
Figure 4.
Hydrogels containing covalently immobilized PDGF-BB enhances vascular network formation in the mouse cornea model compared to hydrogels encapsulating soluble PDGF-BB only [103]. Copyright 2011, with permission from Elsevier.
While the direct attachment of growth factors to scaffolds has enhanced angiogenic response by endothelial cells, one potential limitation of covalently attaching growth factor receptor-binding molecules to biomaterials is that they may prevent receptor internalization or dimerization [104, 105]. In one extreme example, VEGF-mediated endothelial network formation in Matrigel was inhibited by an immobilized QK peptide that could not be released from the surrounding environment, and the putative mechanism involved “receptor pinning” by immobilized QK [106]. Numerous other strategies have been used to modulate soluble growth factor signaling dynamics via strategies that mimic in-vivo mechanisms. The in-vivo ECM is capable of passive and cell-mediated release of soluble growth factors including VEGF [56], bFGF [57], and other pro-angiogenic growth factors. The ECM is also capable of enhancing growth factor stabilization and concentration in the matrix via growth factor-binding glycosaminoglycans and proteoglycans (e.g. heparin) [56–59]. Strategies to mimic relevant ECM-growth factor interactions have been extensively reviewed elsewhere, and include temporal control over growth factor release [100], spatial control over growth factor gradients [107], and inclusion of growth-factor binding and sequestering molecules to the matrix [101, 108].
Mechanical Properties
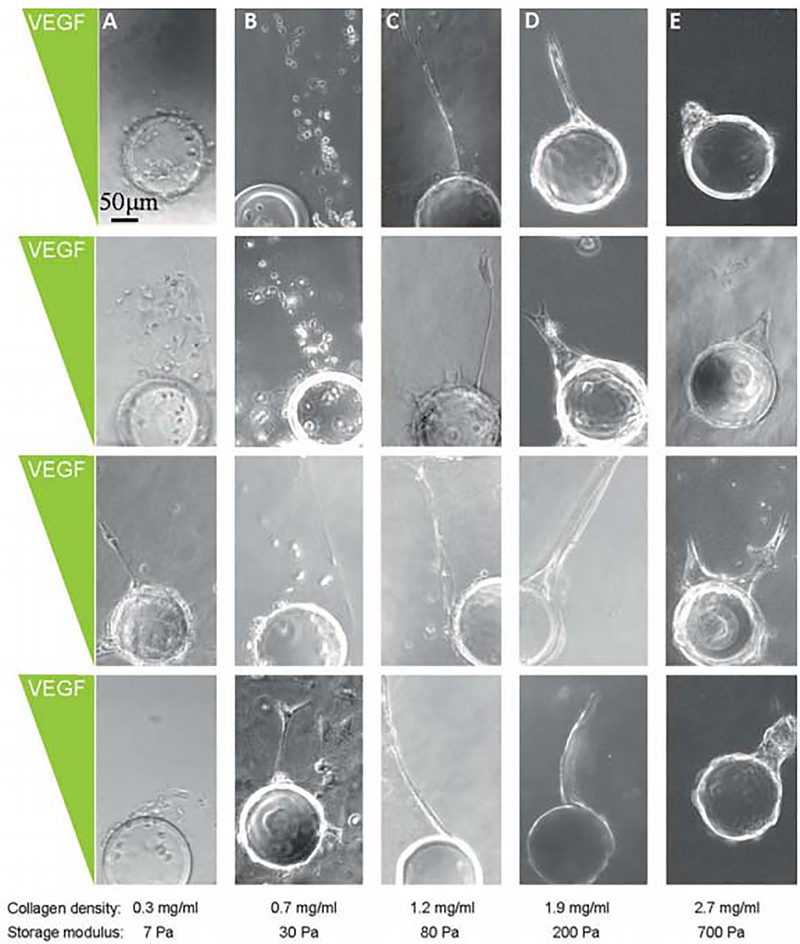
The stiffness of the cellular microenvironment is a critical mediator of cell phenotype, and is a distinguishing feature when comparing normal and diseased (e.g. cancerous) tissues [46, 109]. Optimized stiffness ranges can enable endothelial cell network formation in 2D and 3D environments. For example compliant (elastic modulus 140 Pa) polyacrylamide hydrogels functionalized with 0.1 mM RGD promoted formation of endothelial cell networks while stiffer hydrogels (elastic modulus 2500 Pa) promoted formation of confluent endothelial cell sheets [110]. On collagen-coated polyacrylamide hydrogels, stiffness dictated the expression of pro-angiogenic genes as well as pro-osteogenic genes in HUVECs. Specifically, VEGFR2 gene expression was upregulated on 3 kPa elastic modulus hydrogels, while angiogenic and osteogenic genes were upregulated on 30 kPa elastic modulus hydrogels [111]. In 3D environments a balance between matrix degradability and stability is required to foster HUVEC network formation. One study putatively demonstrated a need for degradable matrices that permit remodeling and cell migration, but retain enough stability to prevent the collapse of a forming vascular network [49]. Interestingly, HUVECs in 3D environments have variable responses to drug treatment depending on the surrounding stiffness and the presence of tumor-derived growth factors. Specifically, HUVECs in one study were more sensitive to the angiogenesis inhibitor Vandetenib when seeded on softer materials than stiffer materials, and treatment with tumor-derived growth factors removed stiffness effects on HUVEC network formation and decreased drug sensitivity [112]. Finally, the density of a hydrogel network also affects endothelial cell responses to VEGF gradients. Specifically, enhanced collagen density increased human dermal microvascular endothelial sprout polarization toward increasing concentrations of VEGF and increased sprout stability (Fig. 5) [53].
Figure 5.
Human dermal microvascular endothelial cell sprouting behavior in VEGF gradients. Sprouting varies depending on collagen density and stiffness [53]. Copyright 2010, with permission from the Royal Society of Chemistry.
Hydrogel stiffness can be tuned by changing polymer concentration [113], crosslinking density [49, 109, 114, 115], and temporal control of stiffness may be controlled using engineered degradation mechanisms [116–118] in most materials. Other strategies have recently changed mechanical properties while also decoupling polymer network density and mechanical stiffness. Collagen modification with tyramine groups and treatment with peroxidases have enabled covalent crosslinking of Collagen hydrogels while still enabling cell-ECM interactions [119], and the crosslinking density modulated network formation by ECFCs. Additionally, the impact of changing stiffness via crosslinking density independently of Collagen density was explored by crosslinking Collagen after non-enzymatic glycation [55, 109]. Increased matrix stiffness increased the extent of capillary sprouting from bovine arterial endothelial cell and HUVEC spheroids, increased sprouting in mouse and chick embryo models, and increased blood vessel permeability. These techniques allowed investigators to explore the effects of changing mechanics without simultaneously affecting the density of structural molecules and insoluble signaling molecules presented to cells.
An additional strategy for controlling material stiffness is the use of biocomposites, in which reinforcing materials such as ceramics and glasses may be added to otherwise soft materials. One study incorporated hydroxyapatite nanocrystals into Fibrin matrices to increase angiogenic sprout number, length and invasion speed by HUVECs [120]. Another study enhanced angiogenic sprouting in aortic ring assays by adding magnesium-doped bioglass to poly(butylene succinate) hydrogels [121]. In both cases, the use of reinforcing materials in the biocomposites was implicated not only in increased stiffness of a pro-angiogenic material, but enhanced growth factor binding and sequestration as well. As such, the mechanical and biochemical properties of biocomposites have the potential to recapitulate angiogenic environments found in bone-related diseases such as bone cancer and ectopic tissue mineralization [122, 123].
Previous studies have demonstrated that degradability is critical to enabling endothelial network formation in 2D [124] and 3D [113] endothelial cell cultures. Many PEG-based hydrogels are crosslinked into a polymer network through the use of cell-degradable crosslinking molecules. Hydrogels are often fully or partially crosslinked using pegylated mimics of a MMP-labile site on Collagen, and the sequence may be modified to enhance or inhibit degradability. For example, the native MMP-labile amino acid sequence of Gly-Ile-Ala-Gly (GIAG) can be exchanged for Gly-Ile-Trp-Gly (GIWG) for enhanced degradability or Gly-Pro-Ala-Gly (GPAG) for inhibited degradability. These changes have been shown to affect the rate of endothelial cell sprouting in an aortic arch assay [125]. Another strategy to modulate degradability is the use of crosslinking peptides with a single MMP-labile site versus peptides with multiple MMP-labile sites, and this strategy has been used to dictate HUVEC migration [126].
To further define the shape of endothelial cell networks and directionality of angiogenic sprouting in biomaterials, modulus and degradability can be spatially defined using innovative techniques. For example, perfusion-based frontal polymerization – the uneven photopolymerization of hydrogels as a result of a concentration gradient of Eosin Y photoinitiator – generates hydrogels with graded density that can dictate the direction of growth by sprouting endothelial cells [127]. Spatially patterned degradability has also been achieved via multiple other methods. Degradability in hyaluronic acid hydrogels was defined by adding MMP-labile crosslinking groups. In addition, a secondary means of regulating degradability was achieved via UV photopolymerization of acrylate-functionalized hyaluronic acid to make certain areas of the hydrogel impassible, thereby dictating locations of ECFC vessel formation [128].
Maintenance of Hypoxia
Numerous vascular disorders lead to decreased levels of dissolved oxygen present in surrounding tissue. This is known to significantly affect endothelial cell phenotype [129], including via Hypoxia Inhibitory Factor (HIF) signaling. Though tests requiring the establishment of a hypoxic environment can be achieved using specialized incubators and bioreactors with controlled atmospheric conditions, recent work has alternatively used biomaterials to expose cells to hypoxic conditions for prolonged periods of time and generate defined oxygen gradients in endothelial cell culture.
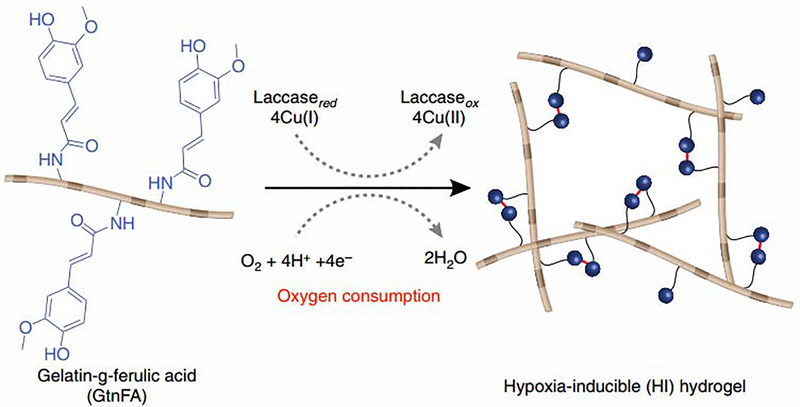
One study achieved control of dissolved oxygen concentrations in gelatin-based materials by including a ferulic acid crosslinking molecule and initiating a crosslinking reaction with Laccase (Fig. 6). The crosslinking reaction consumes oxygen and maintains low oxygen conditions for up to 1 hour after polymerization. This caused increased expression of HIF1α and HIF2α in ECFCs, both of which promote vascular network formation [130]. This Laccase-based crosslinking mechanism was adapted in Dextran-based hydrogels in which oxygen consumption was achieved during crosslinking of tyramine-functionalized dextran. Here, hypoxic conditions in the hydrogels were maintained for up to 12 hours in the hydrogels, and the duration of hypoxia was tunable through polymer concentration and stiffness [131]. Interestingly, materials that regulate dissolved oxygen levels in endothelial cell culture represent an adaptable approach to regulating the cellular microenvironment without the need for bioreactors or similar instrumentation.
Figure 6.
Hydrogel crosslinking reactions can consume oxygen and reduce dissolved oxygen concentrations present in endothelial cell culture environments [130]. Copyright 2014. Used with permission from Nature.
Vascular morphogenesis in co-culture systems
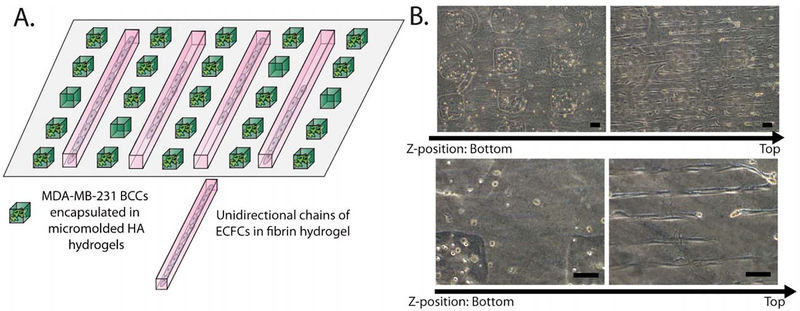
Endothelial cells in tumors interact with cancer cells and cancer stem cells, which respond to environmental cues and subsequently affect the vascular network through physical contact and paracrine signaling [132, 133]. Studies utilizing biomaterials to define populations of endothelial-cancer cell co-cultures and affect phenotypes of heterotypic cell populations are beginning to be applied toward modeling cancer vasculature. For example, discrete spatial patterning of heterotypic cell populations has been achieved through micro-contact printing and cell culture in microfluidic channels. Segregation of breast cancer cells in hyaluronic acid hydrogels from ECFCs in fibrin hydrogels was performed using sequential micro-contact printing (Fig. 7) [134]. Discrete patterning of HUVECs and HeLa cancer cell populations was recently achieved in 2D cell culture by combining micro-contact printing, activation of un-patterned PVA with dopamine, and sequential cell seeding [135]. The separation of cell populations is a promising tool for studying juxtacrine and paracrine control of angiogenesis by cancer cells, as demonstrated in microfluidic platforms. In one example, salivary gland adenoid cystic carcinoma cells and oral squamous cell carcinoma cells induced – to varying degrees based on cell type – sprouting capillary growth by HUVECs through a basement membrane extract matrix [136]. Taken together, these technologies create environments that incorporate relevant ECM cues, as well as endothelial and tumor cells to accurately model the in-vivo tumor environment.
Figure 7.
Spatially segregated co-culture of endothelial cells and cancer cells is achieved by sequential micro-contact printing steps. Materials may also be patterned to induce multicellular structure formation with desired morphologies and geometries [134]. Copyright 2012. Used with permission from the Royal Society of Chemistry.
To recapitulate increasingly complex cancer environments for drug discovery, studies have leveraged materials-based techniques to generate vascularized microtumors [30, 137]. Many vascularized microfluidic tumor models consisted of cancer cells encapsulated in fibrin that were grown directly in co-culture with endothelial cells [138, 139], or spatially partitioned from a neighboring population of endothelial cells in microfluidic channels [140]. These systems demonstrated perfusable vascular networks growing within and around the tumor spheroids [139], as well as cancer cells migrating to and infiltrating vascular networks [140]. In many cases, cancer cells were partitioned inside alginate-based microcapsules to grow in their own isolated environments [141–144]. Alginate capsules are resistant to cell attachment but are also permeable to growth factors such as MCF-7 breast cancer cell-secreted VEGF and HIF1α [141]. The vascularized microtumor model was further developed through the incorporation of alginate capsules containing MCF-7 cells into vascularized Collagen-I hydrogels. Briefly, MCF-7 cells were encapsulated in Collagen-I cores surrounded by alginate shells. The fully vascularized tumor model was generated by encapsulating multiple MCF-7 capsules along with HUVECs and human adipose-derived stem cells into a larger Collagen-I hydrogel [145]. Here, further steps were taken to control biomaterial properties to modulate angiogenesis. The stiffness of the collagen core of the MCF-7 capsules modulated proliferation and expression of vimentin and CXCR7 by the MCF-7 cells. Additionally, direct contact between endothelial cells and MCF-7 cells was controlled through the addition of sodium citrate, which could dissolve alginate shells while leaving the overall tumor model intact. This enabled observation of paracrine signaling or direct cell-cell contact within the model. This modular approach shows promise in allowing for heterogenous biomaterial constructs that can modulate cancer vasculature formation in drug screening models.
PERSPECTIVE: MATERIAL APPLICATIONS TO MODEL CANCER VASCULATURE
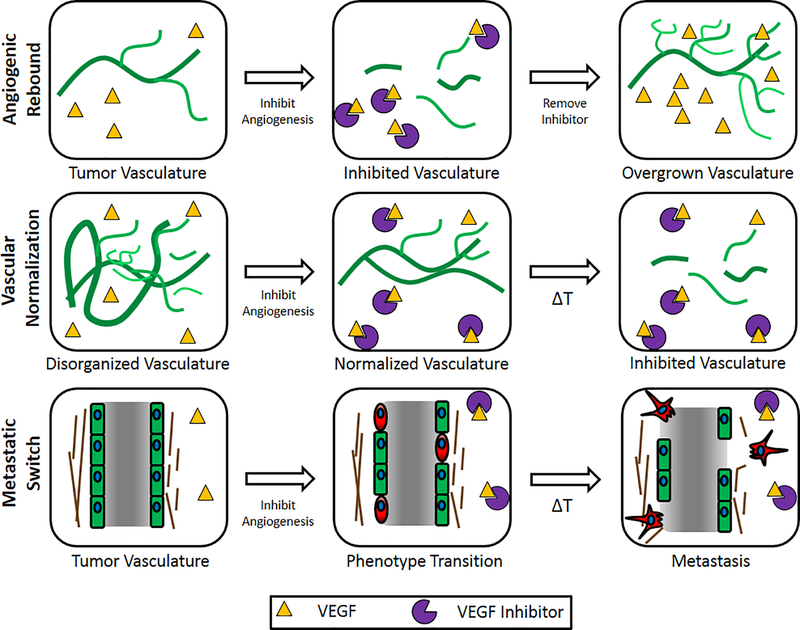
A critical limitation of in-vitro models of human vasculature, particularly those used to identify anti-angiogenic drugs for cancer treatment, is that they only model the effects of initial vascular disruption and are unable to model unintended side effects and long-term effects of treatment. The studies reviewed in the current manuscript represent a toolset that may be applied toward modeling long-term effects of anti-angiogenic drug treatments, including angiogenic rebound, vascular normalization, and the metastatic switch (Fig. 8, Table 2). In this section we speculate on how customizable biomaterials, along with endothelial cells of pathological origin, can be applied toward the creation of advanced in-vitro models of anti-angiogenesis treatment.
Figure 8.
Schematics of Angiogenic Rebound, Vascular Normalization, and Metastatic Switch events after anti-angiogenesis treatment. Angiogenic Rebound is described as a regrowth in cancer vasculature due to restoration of angiogenic growth factor signaling after the cessation of angiogenesis inhibition. Vascular Normalization is described as the brief reorganization of dysfunctional cancer vasculature into well-organized, patent vasculature over the course of angiogenesis inhibition. The Metastatic Switch is described as the phenotypic transformation of numerous cell types in a cancer tumor, including endothelial cells, into highly invasive, migratory cells as a result of angiogenesis inhibition.
Table 2.
Summary of biomaterials engineering tools and their applications in modeling pathological vasculature.
| Biological Context | ||||||||
|---|---|---|---|---|---|---|---|---|
| Biomaterial Design Parameter | Technique | References | Angiogenic Rebound | References | Vascular Normalization | References | Endothelial-Mesenchymal Transition | References |
| Integrin-binding Laminin and Fibronectin analogs | - Covalent binding of adhesion peptides | 49, 79, 80, 82, 83, 86, 87–89 | Environment promotes cell attachment, spreading, tubulogenesis and migration before and after anti-angiogenic treatment | 47–52, 60, 61, 65 | Integrin binding molecules promote lumen formation and stability of patent vascularture during anti-angiogenic treatment | 51, 52, 60, 61, 65 | Cell adhesion to ECM modulates propensity of EndMT | 160, 161 |
| - Covalent binding of full-length ECM proteins | 90 | |||||||
| Spatial patterning of integrin-binding ligands and growth factors | - Photopatterning and liquid immersion patterning | 84, 85, 93, 97, 127, 128 | Vascular networks are tortuous and disorganized prior to anti-angiogenic treatment | 11, 17, 28, 29 | Tortuous and disorganized vascular networks will be converted to organized, patent vasculature during anti-angiogenesis treatment. | 11, 17, 28, 29, 31, 49 | Localization of endothelial cells to defined areas and promotion of cell-cell contacts modulates susceptibility to EndMT | 162, 163 |
| Juxtacrine and Paracrine signaling between cancer cells and endothelial cells, off-target drug effects | - Microcontact printing, segregation of co-cultured cancer cells and endothelial cells | 134–136 | Cancer cells and stromal cells increase production of pro-angiogenic growth factors following anti-angiogenic treatment | 18, 21–23, 132, 133 | Cancer cells and stromal cells secrete pro-angiogenic growth factors during anti-angiogenic treatment, modifying drug dose ranges and schedules necessary for normalization | 23, 132, 133 | Cancer cells and stromal cells secrete cytokines that mediate EndMT | 163, 164 |
| Endothelial cells predisposed to mimick tumor angiogenesis | - Patient-specific endothelial cells | 149 | Endothelial cells are more proliferative, migratory, and less likely to form stable, patent vasculature | 17, 149, 150 | Endothelial cells are more proliferative, migratory, and less likely to form stable, patent vasculature. Anti-angiogenic treatment would enable normalization | 17, 149, 150 | Endothelial cells with prolonged exposure to cancer-derived growth factors and inflammatory growth factors have increased susceptibility to EndMT | 20, 26, 27 |
| - Endothelial cells exposed to cancer-derived conditioned media/inflammatory growth factors | 150 | |||||||
| Elevated growth factor presence, enhanced growth factor stability | - Covalent incorporation of full-length growth factors | 84, 96, 97, 102, 103 | Elevated presence and stability of growth factors causes resistance to drug treatments, contributes to rebound following anti-angiogenic treatment | 18, 21–23 | Increased persistence and stability of growth factors in the microenvironment impacts drug dose rates ane schedules that normalize vasculature | 18, 21–23 | Balance of VEGF, TGFβ, bFGF and other growth factors critical to determining Endothelial Cell susceptibility to EndMT | 159 |
| - Covalent incorporation of peptide growth factor mimicks | 85, 92–95 | |||||||
| - Incorporation of non-covalent growth factor binding ligands | 59, 100, 101, 107, 108 | |||||||
| Sustained hypoxia | - Use of crosslinking reactions that consume oxygen | 130, 131 | Hypoxic environment present in tumors due to dysfunctional vasculature, hypoxic environment sustained following anti-angiogenic treatment | 11, 17, 21–23 | Paracrine signaling from cancer cells are modulated by hypoxia in the tumor environment. This will in turn impact drug dose rates and schedules that normalize vasculature | 11, 17, 21–23 | Hypoxia and HIF1α are implicated as initiators of EndMT | 11, 17, 21–23, 165–169 |
| Elevated stiffness, enhanced environmental stability | - Polymer concentration | 49, 53, 110–113 | Cell culture material persists after anti-angiogenic treatment, may be repopulated by new vasculature | 49, 53–55, 60–65 | Environment promotes lumen formation and structurally supports patent vasculature during normalization | 49, 53–55, 60–65 | Endothelial cells on stiffer matrices more susceptible to EndMT via TGFB signaling | 171 |
| - Crosslinking concentration | 49, 109, 114, 115 | |||||||
| - Degradation | 113, 116–118, 124–126 | |||||||
| - Decoupled stiffness | 55, 109, 119, | |||||||
| - Biocomposites | 120, 121 | |||||||
Generating Pathological Vasculature
To model side effects of anti-angiogenic drug treatment, it will be necessary to generate diseased vasculature, perhaps by using endothelial cells from known pathological origins. These cell types, which may be derived from induced pluripotent stem cells or directly harvested from diseased tissue, can display diseased phenotypes in vascular models prior to exposure to the model environment. For example, endothelial network formation was recently modeled when endothelial cells were derived from induced pluripotent stem cells (IPSCs) of type 1 diabetic patients [146], wherein diabetic patient-derived endothelial cells demonstrated a more severe response to hypoxic conditions [130] compared to non-diabetic endothelial cells. IPSC differentiation protocols can generate endothelial cells from patients with other conditions (e.g. cancer) using a similar approach, which makes iPSC-ECs a particularly valuable tool. Recent studies show that iPSC-ECs can form capillary-like networks on 2D hydrogel surfaces [80] and while encapsulated in 3D hydrogels [146–148].
Primary endothelial cells have been derived from hepatocellular carcinomas and exhibited properties such as enhanced migration, resistance to apoptosis and the ability to form more dense networks of tubules and sprouting capillaries compared to normal endothelial cells [149]. In addition, endothelial cells with prolonged exposure to pathological environments have demonstrated an ability to exacerbate tissue pathology. As an example, human umbilical vein endothelial cells (HUVECs) treated with conditioned media generated from lung carcinoma cell lines and inflammatory growth factors TNFα, VEGF, and bFGF were shown to activate pro-metastatic signaling pathways in lung carcinoma cells [150]. Taken together, these studies demonstrate the potential value of implementing cell types of pathological origin when modeling the effects of anti-angiogenesis treatment.
Several challenges bar the generation of vascular features that are unique to cancer vasculature: abnormally large, tortuous and leaky blood vessels such as mother vessels and vascular malformations; disorganized, hyperpermable and hyperproliferative vessels such as glomeruloid microvascular proliferations; transluminal bridges that travel through larger blood vessels; blood vessels that have variable, rather than constant diameter and wall thickness (Figure 1) [11, 12, 17, 28–30]. Numerous biomaterial properties may be leveraged in tumor models to control the generation of cancer vasculature. For example, blood vessel tortuosity is predicted to be the product of unusually high integrin binding to a heterogeneous ECM [11, 12, 17]. Integrin binding molecules may be patterned heterogeneously into materials to provide anchor points that restrict natural blood vessel lengthening with growth, and therefore generate vessel tortuosity. In a separate example, imbalanced growth factor signaling from the surrounding environment may lead to outcomes such as endothelial hyperproliferation, transluminal bridge formation, loosening of endothelial cell-cell interconnections and abnormal lumen polarity [11, 17]. Imbalanced growth factor signaling may be recapitulated by co-culture with growth factor-secreting fibroblasts and cancer cells, as well as designing materials that lack the ability to bind to growth factors in ways that generate stable growth factor gradients [17]. Growth factor presence in the environment should be at higher sustained concentrations than normal, and should not directionally guide blood vessel growth in a systematic way. To mediate the formation of abnormally large blood vessels, chosen culture materials should be extensively degradable in order to permit blood vessel widening [11, 17]. Heterogeneity throughout the material may also be used to resemble the heterogeneity of tumor microenvironments and encourage the formation of blood vessels with changing diameter at different points of a vessel [12].
Angiogenic Rebound
Angiogenic rebound is the excessive reassembly of vascular networks that were initially disrupted by anti-angiogenic drug treatment [18, 21–23]. Modeling this phenomenon requires the ability to sustain cell adhesion, viability and migration after anti-angiogenic drug treatment. Biomaterial customization approaches reviewed here may be leveraged to add adhesion ligands such as YIGSR, IKVAV and RGD to chemically-defined hydrogels to improve cell survival after initial network disruption. Materials may also be tailored for culture of iPSC-ECs and cancer-derived endothelial cells to address a hypothesis that pathological origins may improve endothelial cell drug resistance and angiogenic rebound (Table 1).
One cause of angiogenic rebound is the elevated quantity of angiogenic growth factors secreted by fibroblasts and cancer cells into the extracellular environment after anti-angiogenic treatment [18, 21–23]. These effects can potentially be recapitulated by using photopolymerizable hydrogels and sequential microprinting techniques to culture segregated populations of endothelial cells and growth factor-secreting cells. The addition of growth factor-binding ligands to a biomaterial may also maintain an elevated presence of growth factors for long periods of time. Additionally, the use of hydrogels with hypoxia-induced crosslinking would result in temporary maintenance of hypoxia and consequent upregulation of endogenous growth factor secretion in the model tumor environment. Successful implementation of these features potentially leads to models in which angiogenic rebound is observable in time frames resembling those observed in vivo, and mechanisms may be targeted to prevent network reformation in tumors.
Vascular Normalization
Vascular normalization is the transformation of dysfunctional vasculature into more stable, patent vasculature following short-term anti-angiogenic drug treatment [11, 17, 21–23, 28, 29, 31]. Modeling this phenomenon in vitro may require vascular network features such as network area and stability to be quantifiable as potential markers of normalization events. Increased biomimicry of vascular models which recapitulate aspects of the in-vivo environment such as pharmacokinetics [151] and juxtacrine/paracrine signaling between various cancer, stromal, and endothelial cell types [23, 132, 133] will determine meaningful drug concentration ranges and dosing timelines that predictably trigger normalization. Initial pathological network formation may be achieved through the use of cancer-derived endothelial cells in order to generate disorganized and disrupted vasculature prior to exposure to anti-angiogenic drugs [17, 149]. Studies of vascular normalization may need to be done in materials wherein anti-angiogenic drugs can either improve or disrupt vascular network stability depending on drug concentration drug doses. For example, we previously developed 3D models of endothelial cell network formation in which a hydrogel with low modulus and high RGD concentration caused the VEGF inhibitor SU5416 to stabilize vascular networks rather than disrupt vascular network formation [49]. Finally, customizable materials may need to be adapted for systems that permit fluid perfusion through model vasculature in order to observe the impact of drug treatments on vessel leakiness and stability. Successful generation of normalization models may reveal appropriate dose ranges, time tables and biomarkers to recognize and control normalization in vivo.
Metastatic Switch and Mesenchymal Transition
An important side effect associated with anti-angiogenic drug treatment, specifically the inhibition of VEGF signaling, is the onset of pathological phenotypes in endothelial cells. One example of this phenotypic switch is the Endothelial-Mesenchymal Transition (EndMT), where endothelial cells change phenotypes to resemble mesenchymal cells [122, 152–156] that contribute to cancer growth and metastasis [26, 27, 157]. EndMT has been shown to be dependent on multiple environmental factors, notably TGF-β signaling. Evidence has emerged to demonstrate that inhibiting VEGF signaling by anti-angiogenic treatment increases migratory phenotypes in endothelial cells and cancer cells in mouse models [20], as VEGF is known to attenuate TGF-β activity [158].
The identification of enhanced cancer metastasis as a side effect of angiogenic inhibition highlights an urgent need to characterize the effects of VEGF inhibition in tumor-like environments that influence endothelial responses to TGF-β. These environmental variables include TGF-β concentration [159], integrin interactions with the ECM [160, 161] and β-catenin [162], cell-cell contacts [163], and co-cultures with supporting cell types [164]. Recent evidence also implicates hypoxia as an inducer of EndMT through SNAIL and Smad2/3 activation [165–169]. The effects of specific interactions between endothelial cells and the ECM on EndMT are only beginning to be answered [170]. In one study, cell-adhesive poly(L-Lysine)/hyalouronic acid films increased the propensity of HUVECs to undergo TGF-β1-mediated EndMT on materials with increasing stiffness [171]. Results such as these begin to further implicate pathological extracellular environments as agonists of EndMT. Heparin binding molecules [59, 108] may be another important component to add to EndMT models, as they facilitate binding, concentration and stabilization of soluble TGF-β locally near endothelial cell populations. Other ligands, including PHSRN peptide, act synergistically with RGD in full-length fibronectin to potentially mediate EndMT as well [172]. A greater understanding of ECM-modulation of EndMT may include discovery of appropriate drug dosing ranges for achieving vascular disruption while avoiding EndMT, as well as elucidation of therapeutic targets to prevent the transition.
Anti-angiogenic drug discovery
The generation of physiologically-relevant tumor models will be critical toward designing and improving therapeutics that overcome the limitations of current technologies (Table 1). Future drug discovery models will be required to mimick vascular features that act as known limitations that diminish the efficacy of cancer therapeutics [30]. Lowered drug efficacy is largely due to limited access of drugs to critical blood vessels in the tumor [11, 17]. Barriers to access include poor blood perfusion by cancer vasculature and the binding site barrier (accumulation of drugs at the exterior areas of a tumor due to binding to target molecules) [30]. Lowered drug efficacy can also result from drug resistance of the endothelial cells themselves, either due to aberrant gene expression [10, 13], and an unbalanced growth factor signaling environment maintained by cancer and perivascular cell types [11].
Consequently, two critical elements of simulating the above limitations are perfusion of model blood vessels, and comprehensive monitoring of marker expression changes by endothelial cells during and following anti-angiogenic drug treatment. Materials techniques described here would contribute to the generation of cancer-like, perusable vasculature that would allow scientists to track the penetration of drugs into a tumor, and whether treatment normalizes vasculature. Because tumors differ biologically from one another [30], the use of cancer cells and endothelial cells derived from tumors would be ideal for vetting potential therapeutics. Most cancer models continue to use HUVECs as endothelial components of tumor models, and future models should begin to use cancer-derived or patient-derived endothelial cells for the benefit of targeting markers and signaling pathways that are specific to separate tumor types. The use of combined cancer cells and endothelial cells in microtumor models has been used to test both anti-cancer and anti-angiogenic drugs on both cell types simultaneously [139, 145], and these would be ideal platforms for testing the effects of combined drug delivery for cancer treatment. The application of these models following failed clinical trials can rapidly improve the efficacy of drugs that would have otherwise been successful. Similarly, cancer models developed as screening tools can determine therapeutics that overcome known barriers early, then see validation in animal models and clinical trials [30]. Finally, successful modeling of therapeutic limitations as well as growing knowledge on drug interactions with materials may bring insight into materials-mediated drug delivery techniques. Existing biomaterials-mediated drug delivery techniques, such as transport of micro-and-nanoparticles through tumors, and MMP-based drug release from ECM-derived materials are reviewed in greater detail elsewhere [30, 173, 174].
CONCLUSION
Recently developed technologies in biomaterials development are leading to unprecedented control over in-vitro endothelial cell culture environments as well as the ability to model mechanisms of normal and pathological angiogenesis. With the systematic utilization of the techniques reviewed here, models of cancer vasculature will likely improve understanding of far-reaching side effects of anti-angiogenic treatment, which may translate into improvements in cancer therapy.
ACKNOWLEDGEMENTS
The authors would like to acknowledge funding from the National Institutes of Health (NIH R01HL093282-01A1, R21EB016381-01, 1UH2TR000506-01, and the Biotechnology Training Program NIGMS5T32GM08349), the UW-Madison Graduate Engineering Research Scholars program, the Environmental Protection Agency (STAR grant no. 83573701), the UW-Madison Molecular and Environmental Toxicity Center Training Program (NIH T32 ES007015). This review was supported by the National Institutes of Health, under Ruth L. Kirschstein National Research Service Award T32 HL 007936 from the National Heart Lung and Blood Institute to the University of Wisconsin-Madison Cardiovascular Research Center.
Footnotes
CONFLICT OF INTEREST STATEMENT
W.L.M. is a founder and stockholder of Stem Pharm.
DATA AVAILABILITY STATEMENT
This review article does not present original data that is not already covered by cited articles.
REFERENCES
- [1].De Smet F, Segura I, De Bock K, Hohensinner P, Carmeliet P, Mechanisms of Vessel Branching Filopodia on Endothelial Tip Cells Lead the Way, Arteriosclerosis Thrombosis and Vascular Biology 29(5) (2009) 639–649. [DOI] [PubMed] [Google Scholar]
- [2].Ribatti D, Judah Folkman, a pioneer in the study of angiogenesis, Angiogenesis 11(1) (2008) 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].FOLKMAN J, LONG DM, BECKER FF, Growth and metastasis of tumor in organ culture, Cancer 16 (1963) 453–67. [DOI] [PubMed] [Google Scholar]
- [4].Wang Y, Fei D, Vanderlaan M, Song A, Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro, Angiogenesis 7(4) (2004) 335–45. [DOI] [PubMed] [Google Scholar]
- [5].Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, Chan E, Moss KG, Haznedar JO, Sukbuntherng J, Blake RA, Sun L, Tang C, Miller T, Shirazian S, McMahon G, Cherrington JM, In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship, Clin Cancer Res 9(1) (2003) 327–37. [PubMed] [Google Scholar]
- [6].De Falco S, Antiangiogenesis therapy: an update after the first decade, Korean J Intern Med 29(1) (2014) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cao Y, Langer R, Optimizing the delivery of cancer drugs that block angiogenesis, Sci Transl Med 2(15) (2010) 15ps3. [DOI] [PubMed] [Google Scholar]
- [8].Samples J, Willis M, Klauber-Demore N, Targeting angiogenesis and the tumor microenvironment, Surg Oncol Clin N Am 22(4) (2013) 629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chung AS, Lee J, Ferrara N, Targeting the tumour vasculature: insights from physiological angiogenesis, Nat Rev Cancer 10(7) (2010) 505–14. [DOI] [PubMed] [Google Scholar]
- [10].Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG, Cancer drug resistance: an evolving paradigm, Nat Rev Cancer 13(10) (2013) 714–26. [DOI] [PubMed] [Google Scholar]
- [11].McDonald DM, Baluk P, Significance of blood vessel leakiness in cancer, Cancer Res 62(18) (2002) 5381–5. [PubMed] [Google Scholar]
- [12].Wu M, Swartz MA, Modeling tumor microenvironments in vitro, J Biomech Eng 136(2) (2014) 021011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hida K, Ohga N, Kurosu T, Totsuka Y, Shindoh M, Crosstalk between Blood Vessels and Tumor Microenvironment, Oral Science International 7(1) (2010) 1–10. [Google Scholar]
- [14].Ruoslahti E, Specialization of tumour vasculature, Nat Rev Cancer 2(2) (2002) 83–90. [DOI] [PubMed] [Google Scholar]
- [15].Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, Mason W, Mikkelsen T, Phuphanich S, Ashby LS, Degroot J, Gattamaneni R, Cher L, Rosenthal M, Payer F, Jürgensmeier JM, Jain RK, Sorensen AG, Xu J, Liu Q, van den Bent M, Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma, J Clin Oncol 31(26) (2013) 3212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ, Mehta MP, A randomized trial of bevacizumab for newly diagnosed glioblastoma, N Engl J Med 370(8) (2014) 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nagy JA, Chang SH, Dvorak AM, Dvorak HF, Why are tumour blood vessels abnormal and why is it important to know?, Br J Cancer 100(6) (2009) 865–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Loges S, Schmidt T, Carmeliet P, Mechanisms of resistance to anti-angiogenic therapy and development of third-generation anti-angiogenic drug candidates, Genes Cancer 1(1) (2010) 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Welti J, Loges S, Dimmeler S, Carmeliet P, Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer, J Clin Invest 123(8) (2013) 3190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lu KV, Chang JP, Parachoniak CA, Pandika MM, Aghi MK, Meyronet D, Isachenko N, Fouse SD, Phillips JJ, Cheresh DA, Park M, Bergers G, VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex, Cancer Cell 22(1) (2012) 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].El-Kenawi AE, El-Remessy AB, Angiogenesis inhibitors in cancer therapy: mechanistic perspective on classification and treatment rationales, Br J Pharmacol 170(4) (2013) 712–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dey N, De P, Brian LJ, Evading anti-angiogenic therapy: resistance to anti-angiogenic therapy in solid tumors, Am J Transl Res 7(10) (2015) 1675–98. [PMC free article] [PubMed] [Google Scholar]
- [23].Maj E, Papiernik D, Wietrzyk J, Antiangiogenic cancer treatment: The great discovery and greater complexity (Review), Int J Oncol 49(5) (2016) 1773–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Seftor RE, Hess AR, Seftor EA, Kirschmann DA, Hardy KM, Margaryan NV, Hendrix MJ, Tumor cell vasculogenic mimicry: from controversy to therapeutic promise, Am J Pathol 181(4) (2012) 1115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ, Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry, Am J Pathol 155(3) (1999) 739–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zeisberg E, Potenta S, Xie L, Zeisberg M, Kalluri R, Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts, Cancer Research 67(21) (2007) 10123–10128. [DOI] [PubMed] [Google Scholar]
- [27].Potenta S, Zeisberg E, Kalluri R, The role of endothelial-to-mesenchymal transition in cancer progression, British Journal of Cancer 99(9) (2008) 1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ma J, Waxman DJ, Combination of antiangiogenesis with chemotherapy for more effective cancer treatment, Mol Cancer Ther 7(12) (2008) 3670–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK, Normalization of the vasculature for treatment of cancer and other diseases, Physiol Rev 91(3) (2011) 1071–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].van den Brand D, Massuger LF, Brock R, Verdurmen WP, Mimicking Tumors: Toward More Predictive In Vitro Models for Peptide- and Protein-Conjugated Drugs, Bioconjug Chem 28(3) (2017) 846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Abdalla AME, Xiao L, Ullah MW, Yu M, Ouyang C, Yang G, Current Challenges of Cancer Anti-angiogenic Therapy and the Promise of Nanotherapeutics, Theranostics 8(2) (2018) 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR, Vianello F, Leblanc P, Munn LL, Huang P, Duda DG, Fukumura D, Jain RK, Poznansky MC, Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy, Proc Natl Acad Sci U S A 109(43) (2012) 17561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jain RK, Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy, Science 307(5706) (2005) 58–62. [DOI] [PubMed] [Google Scholar]
- [34].Cesca M, Bizzaro F, Zucchetti M, Giavazzi R, Tumor delivery of chemotherapy combined with inhibitors of angiogenesis and vascular targeting agents, Front Oncol 3 (2013) 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cesca M, Morosi L, Berndt A, Fuso Nerini I, Frapolli R, Richter P, Decio A, Dirsch O, Micotti E, Giordano S, D’Incalci M, Davoli E, Zucchetti M, Giavazzi R, Bevacizumab-Induced Inhibition of Angiogenesis Promotes a More Homogeneous Intratumoral Distribution of Paclitaxel, Improving the Antitumor Response, Mol Cancer Ther 15(1) (2016) 125–35. [DOI] [PubMed] [Google Scholar]
- [36].Chen BB, Lu YS, Lin CH, Chen WW, Wu PF, Hsu CY, Yu CW, Wei SY, Cheng AL, Shih TT, A pilot study to determine the timing and effect of bevacizumab on vascular normalization of metastatic brain tumors in breast cancer, BMC Cancer 16 (2016) 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sarkanen JR, Mannerström M, Vuorenpää H, Uotila J, Ylikomi T, Heinonen T, Intra-Laboratory Pre-Validation of a Human Cell Based in vitro Angiogenesis Assay for Testing Angiogenesis Modulators, Front Pharmacol 1 (2010) 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Faulkner A, Purcell R, Hibbert A, Latham S, Thomson S, Hall WL, Wheeler-Jones C, Bishop-Bailey D, A thin layer angiogenesis assay: a modified basement matrix assay for assessment of endothelial cell differentiation, BMC Cell Biol 15 (2014) 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Evensen L, Micklem DR, Link W, Lorens JB, A novel imaging-based high-throughput screening approach to anti-angiogenic drug discovery, Cytometry A 77(1) (2010) 41–51. [DOI] [PubMed] [Google Scholar]
- [40].Staton CA, Reed MW, Brown NJ, A critical analysis of current in vitro and in vivo angiogenesis assays, Int J Exp Pathol 90(3) (2009) 195–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, Shalinsky DR, Hu-Lowe DD, McDonald DM, Rapid vascular regrowth in tumors after reversal of VEGF inhibition, J Clin Invest 116(10) (2006) 2610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G, Yancopoulos GD, McDonald DM, Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts, Am J Pathol 165(1) (2004) 35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bergers G, Hanahan D, Modes of resistance to anti-angiogenic therapy, Nat Rev Cancer 8(8) (2008) 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jakobsson L, Claesson-Welsh L, Vascular basement membrane components in angiogenesis-an act of balance, ScientificWorldJournal 8 (2008) 1246–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM, Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors, Am J Pathol 163(5) (2003) 1801–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fang M, Yuan J, Peng C, Li Y, Collagen as a double-edged sword in tumor progression, Tumour Biol 35(4) (2014) 2871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Davis G, Senger D, Endothelial extracellular matrix - Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization, Circulation Research 97(11) (2005) 1093–1107. [DOI] [PubMed] [Google Scholar]
- [48].Weis SM, Cheresh DA, αV integrins in angiogenesis and cancer, Cold Spring Harb Perspect Med 1(1) (2011) a006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nguyen EH, Zanotelli MR, Schwartz MP, Murphy WL, Differential effects of cell adhesion, modulus and VEGFR-2 inhibition on capillary network formation in synthetic hydrogel arrays, Biomaterials 35(7) (2014) 2149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Senger DR, Perruzzi CA, Cell migration promoted by a potent GRGDS-containing thrombin-cleavage fragment of osteopontin, Biochim Biophys Acta 1314(1–2) (1996) 13–24. [DOI] [PubMed] [Google Scholar]
- [51].Zovein A, Luque A, Turlo K, Hofmann J, Yee K, Becker M, Fassler R, Mellman I, Lane T, Iruela-Arispe M, beta 1 Integrin Establishes Endothelial Cell Polarity and Arteriolar Lumen Formation via a Par3-Dependent Mechanism, Developmental Cell 18(1) (2010) 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bayless KJ, Salazar R, Davis GE, RGD-dependent vacuolation and lumen formation observed during endothelial cell morphogenesis in three-dimensional fibrin matrices involves the alpha(v)beta(3) and alpha(5)beta(1) integrins, Am J Pathol 156(5) (2000) 1673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].S. A., H.S. C., Matrix density mediates polarization and lumen formation of endothelial sprouts in VEGF gradients, Lab on a Chip 10 (2010) 3061–3068. [DOI] [PubMed] [Google Scholar]
- [54].Sun G, Shen YI, Kusuma S, Fox-Talbot K, Steenbergen CJ, Gerecht S, Functional neovascularization of biodegradable dextran hydrogels with multiple angiogenic growth factors, Biomaterials 32(1) (2011) 95–106. [DOI] [PubMed] [Google Scholar]
- [55].Mason B, Starchenko A, Williams R, Bonassar A, Reinhart-King C, Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior, Acta Biomaterialia 9 (2013) 4635–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wijelath E, Namekata M, Murray J, Furuyashiki M, Zhang S, Coan D, Wakao M, Harris R, Suda Y, Wang L, Sobel M, Multiple Mechanisms for Exogenous Heparin Modulation of Vascular Endothelial Growth Factor Activity, Journal of Cellular Biochemistry 111(2) (2010) 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Forsten-Williams K, Chua C, Nugent M, The kinetics of FGF-2 binding to heparan sulfate proteoglycans and MAP kinase signaling, Journal of Theoretical Biology 233(4) (2005) 483–499. [DOI] [PubMed] [Google Scholar]
- [58].Smith JC, Hagemann A, Saka Y, Williams PH, Understanding how morphogens work, Philosophical Transactions of the Royal Society B: Biological Sciences 363(1495) (2008) 1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hudalla G, Koepsel J, Murphy W, Surfaces That Sequester Serum-Borne Heparin Amplify Growth Factor Activity, Advanced Materials 23(45) (2011) 5415–5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cebe-Suarez S, Zehnder-Fjallman A, Ballmer-Hofer K, The role of VEGF receptors in angiogenesis; complex partnerships, Cellular and Molecular Life Sciences 63(5) (2006) 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Somanath P, Malinin N, Byzova T, Cooperation between integrin alpha(nu)beta(3) and VEGFR2 in angiogenesis, Angiogenesis 12(2) (2009) 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Mammoto A, Connor K, Mammoto T, Yung C, Huh D, Aderman C, Mostoslavsky G, Smith L, Ingber D, A mechanosensitive transcriptional mechanism that controls angiogenesis, Nature 457(7233) (2009) 1103–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bryan B, Dennstedt E, Mitchell D, Walshe T, Noma K, Loureiro R, Saint-Geniez M, Campaigniac J, Liao J, D’Amore P, RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis, Faseb Journal 24(9) (2010) 3186–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].van Nieuw Amerongen GP, Koolwijk P, Versteilen A, van Hinsbergh VW, Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro, Arterioscler Thromb Vasc Biol 23(2) (2003) 211–7. [DOI] [PubMed] [Google Scholar]
- [65].Ingber DE, Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology, Circ Res 91(10) (2002) 877–87. [DOI] [PubMed] [Google Scholar]
- [66].Smithmyer ME, Sawicki LA, Kloxin AM, Hydrogel scaffolds as in vitro models to study fibroblast activation in wound healing and disease, Biomater Sci 2(5) (2014) 634–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cushing MC, Anseth KS, Materials science. Hydrogel cell cultures, Science 316(5828) (2007) 1133–4. [DOI] [PubMed] [Google Scholar]
- [68].Murphy WL, McDevitt TC, Engler AJ, Materials as stem cell regulators, Nat Mater 13(6) (2014) 547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Murrow LM, Weber RJ, Gartner ZJ, Dissecting the stem cell niche with organoid models: an engineering-based approach, Development 144(6) (2017) 998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Cruz-Acuna R, Garcia AJ, Synthetic hydrogels mimicking basement membrane matrices to promote cell-matrix interactions, Matrix Biol 57-58 (2017) 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lutolf MP, Hubbell JA, Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering, Nature Biotechnology 23(1) (2005) 47–55. [DOI] [PubMed] [Google Scholar]
- [72].Nuttelman CR, Henry SM, Anseth KS, Synthesis and characterization of photocrosslinkable, degradable poly(vinyl alcohol)-based tissue engineering scaffolds, Biomaterials 23(17) (2002) 3617–3626. [DOI] [PubMed] [Google Scholar]
- [73].Schmedlen KH, Masters KS, West JL, Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering, Biomaterials 23(22) (2002) 4325–4332. [DOI] [PubMed] [Google Scholar]
- [74].Zustiak SP, Wei Y, Leach JB, Protein-hydrogel interactions in tissue engineering: mechanisms and applications, Tissue Eng Part B Rev 19(2) (2013) 160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Sun J, Tan H, Alginate-Based Biomaterials for Regenerative Medicine Applications, Materials 6 (2013) 1285–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lévesque SG, Shoichet MS, Synthesis of cell-adhesive dextran hydrogels and macroporous scaffolds, Biomaterials 27(30) (2006) 5277–85. [DOI] [PubMed] [Google Scholar]
- [77].Lévesque SG, Lim RM, Shoichet MS, Macroporous interconnected dextran scaffolds of controlled porosity for tissue-engineering applications, Biomaterials 26(35) (2005) 7436–46. [DOI] [PubMed] [Google Scholar]
- [78].Ruoslahti E, RGD and other recognition sequences for integrins, Annu Rev Cell Dev Biol 12 (1996) 697–715. [DOI] [PubMed] [Google Scholar]
- [79].Bellis SL, Advantages of RGD peptides for directing cell association with biomaterials, Biomaterials 32(18) (2011) 4205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Nguyen E, Daly W, Le NN, Farnoodian M, Belair D, Schwartz M, Lebakken C, Ananiev G, Saghiri MA, Knudsen T, Sheibani N, Murphy W, Versatile synthetic alternatives to Matrigel for vascular toxicity screening and stem cell expansion, Nature Biomedical Engineering 1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Le Saux G, Magenau A, Gunaratnam K, Kilian K, Bocking T, Gooding J, Gaus K, Spacing of Integrin Ligands Influences Signal Transduction in Endothelial Cells, Biophysical Journal 101(4) (2011) 764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Shen C, Raghavan S, Xu Z, Baranski J, Yu X, Wozniak M, Miller J, Gupta M, Buckbinder L, Chen C, Decreased cell adhesion promotes angiogenesis in a Pyk2-dependent manner, Experimental Cell Research 317(13) (2011) 1860–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hanjaya-Putra D, Bose V, Shen Y, Yee J, Khetan S, Fox-Talbot K, Steenbergen C, Burdick J, Gerecht S, Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix, Blood 118(3) (2011) 804–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Leslie-Barbick JE, Shen C, Chen C, West JL, Micron-scale spatially patterned, covalently immobilized vascular endothelial growth factor on hydrogels accelerates endothelial tubulogenesis and increases cellular angiogenic responses, Tissue Eng Part A 17(1–2) (2011) 221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Stahl P, Chan T, Shen Y-I, Sun G, Gerecht S, Yu M, Capillary Network-Like Organization of Endothelial Cells in PEGDA Scaffolds Encoded with Angiogenic Signals via Triple Helical Hybridization, Advanced Functional Materials 24 (2014) 3213–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ali S, Saik JE, Gould DJ, Dickinson ME, West JL, Immobilization of Cell-Adhesive Laminin Peptides in Degradable PEGDA Hydrogels Influences Endothelial Cell Tubulogenesis, Biores Open Access 2(4) (2013) 241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Jung JP, Jones JL, Cronier SA, Collier JH, Modulating the mechanical properties of self-assembled peptide hydrogels via native chemical ligation, Biomaterials 29(13) (2008) 2143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Jung JP, Nagaraj AK, Fox EK, Rudra JS, Devgun JM, Collier JH, Co-assembling peptides as defined matrices for endothelial cells, Biomaterials 30(12) (2009) 2400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Jung JP, Moyano JV, Collier JH, Multifactorial optimization of endothelial cell growth using modular synthetic extracellular matrices, Integr Biol (Camb) 3(3) (2011) 185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Munoz-Pinto DJ, Guiza-Arguello VR, Becerra-Bayona SM, Erndt-Marino J, Samavedi S, Malmut S, Russell B, Hook M, Hahn MS, Collagen-mimetic hydrogels promote human endothelial cell adhesion, migration and phenotypic maturation Journal of Materials Chemistry B 3 (2015) 7912–7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].D’Andrea LD, Iaccarino G, Fattorusso R, Sorriento D, Carannante C, Capasso D, Trimarco B, Pedone C, Targeting angiogenesis: structural characterization and biological properties of a de novo engineered VEGF mimicking peptide, Proc Natl Acad Sci U S A 102(40) (2005) 14215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Leslie-Barbick J, Saik J, Gould D, Dickinson M, West J, The promotion of microvasculature formation in poly(ethylene glycol) diacrylate hydrogels by an immobilized VEGF-mimetic peptide, Biomaterials 32(25) (2011) 5782–5789. [DOI] [PubMed] [Google Scholar]
- [93].Lee JS, Wagoner Johnson AJ, Murphy WL, A modular, hydroxyapatite-binding version of vascular endothelial growth factor, Adv Mater 22(48) (2010) 5494–8. [DOI] [PubMed] [Google Scholar]
- [94].Chan T, Stahl P, Yu S, Matrix-Bound VEGF Mimetic Peptides: Design and Endothelial-Cell Activation in Collagen Scaffolds, Advanced Functional Materials 21(22) (2011) 4252–4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Chan T, Stahl P, Li Y, Yu M, Collagen–gelatin mixtures as wound model, and substrates for VEGF-mimetic peptide binding and endothelial cell activation, Acta Biomaterialia 15 (2015) 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Guex AG, Hegemann D, Giraud MN, Tevaearai HT, Popa AM, Rossi RM, Fortunato G, Covalent immobilisation of VEGF on plasma-coated electrospun scaffolds for tissue engineering applications, Colloids Surf B Biointerfaces 123 (2014) 724–33. [DOI] [PubMed] [Google Scholar]
- [97].Oh HH, Lu H, Kawazoe N, Chen G, Spatially guided angiogenesis by three-dimensional collagen scaffolds micropatterned with vascular endothelial growth factor, J Biomater Sci Polym Ed 23(17) (2012) 2185–95. [DOI] [PubMed] [Google Scholar]
- [98].Wells L, Valic M, Lisovsky A, Sefton M, Angiogenic Biomaterials to Promote Tissue Vascularization and Integration, Israel Journal of Chemistry 53 (2013) 637–645. [Google Scholar]
- [99].Moon JJ, West JL, Vascularization of engineered tissues: approaches to promote angio-genesis in biomaterials, Curr Top Med Chem 8(4) (2008) 300–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Lee K, Silva EA, Mooney DJ, Growth factor delivery-based tissue engineering: general approaches and a review of recent developments, J R Soc Interface 8(55) (2011) 153–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Belair DG, Le NN, Murphy WL, Design of growth factor sequestering biomaterials, Chem Commun (Camb) 50(99) (2014) 15651–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Saik JE, Gould DJ, Keswani AH, Dickinson ME, West JL, Biomimetic hydrogels with immobilized ephrinA1 for therapeutic angiogenesis, Biomacromolecules 12(7) (2011) 2715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Saik J, Gould D, Watkins E, Dickinson M, West J, Covalently immobilized platelet-derived growth factor-BB promotes antiogenesis in biomirnetic poly(ethylene glycol) hydrogels, Acta Biomaterialia 7(1) (2011) 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Santos SC, Miguel C, Domingues I, Calado A, Zhu Z, Wu Y, Dias S, VEGF and VEGFR-2 (KDR) internalization is required for endothelial recovery during wound healing, Exp Cell Res 313(8) (2007) 1561–74. [DOI] [PubMed] [Google Scholar]
- [105].Simons M, An inside view: VEGF receptor trafficking and signaling, Physiology (Bethesda) 27(4) (2012) 213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Koepsel JT, Nguyen EH, Murphy WL, Differential effects of a soluble or immobilized VEGFR-binding peptide, Integr Biol (Camb) 4(8) (2012) 914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Nguyen E, Schwartz M, Murphy W, Biomimetic Approaches to Control Soluble Concentration Gradients in Biomaterials, Macromolecular Bioscience 11(4) (2011) 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hudalla GA, Murphy WL, Biomaterials that regulate growth factor activity via bioinspired interactions, Adv Funct Mater 21(10) (2011) 1754–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bordeleau F, Mason BN, Lollis EM, Mazzola M, Zanotelli MR, Somasegar S, Califano JP, Montague C, LaValley DJ, Huynh J, Mencia-Trinchant N, Negrón Abril YL, Hassane DC, Bonassar LJ, Butcher JT, Weiss RS, Reinhart-King CA, Matrix stiffening promotes a tumor vasculature phenotype, Proc Natl Acad Sci U S A 114(3) (2017) 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Saunders R, Hammer D, Assembly of Human Umbilical Vein Endothelial Cells on Compliant Hydrogels, Cellular and Molecular Bioengineering 3(1) (2010) 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Santos L, Fuhrmann G, Juenet M, Amdursky N, Horejs CM, Campagnolo P, Stevens MM, Extracellular Stiffness Modulates the Expression of Functional Proteins and Growth Factors in Endothelial Cells, Adv Healthc Mater (2015). [DOI] [PubMed] [Google Scholar]
- [112].Wu Y, Guo B, Ghosh G, Differential Effects of Tumor Secreted Factors on Mechanosensitivity, Capillary Branching, and Drug Responsiveness in PEG Hydrogels, Ann Biomed Eng 43(9) (2015) 2279–90. [DOI] [PubMed] [Google Scholar]
- [113].Moon JJ, Saik JE, Poche RA, Leslie-Barbick JE, Lee SH, Smith AA, Dickinson ME, West JL, Biomimetic hydrogels with pro-angiogenic properties, Biomaterials 31(14) (2010) 3840–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kloxin AM, Kloxin CJ, Bowman CN, Anseth KS, Mechanical Properties of Cellularly Responsive Hydrogels and Their Experimental Determination, Advanced Materials 22(31) (2010) 3484–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Hansen TD, Koepsel JT, Le NN, Nguyen EH, Zorn S, Parlato M, Loveland SG, Schwartz MP, Murphy WL, Biomaterial arrays with defined adhesion ligand densities and matrix stiffness identify distinct phenotypes for tumorigenic and nontumorigenic human mesenchymal cell types, Biomater Sci 2(5) (2014) 745–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Burdick JA, Murphy WL, Moving from static to dynamic complexity in hydrogel design, Nat Commun 3 (2012) 1269. [DOI] [PubMed] [Google Scholar]
- [117].Deshayes S, Kasko A, Polymeric Biomaterials with Engineered Degradation, Journal of Polymer Science - Polymer Chemistry 51 (2013) 3531–3566. [Google Scholar]
- [118].Ginjupalli K, Shavi GV, Averineni RK, Bhat M, Udupa N, PN U, Poly(α-hydroxy acid) based polymers: A review on material and degradation aspects, Polymer Degradation and Stability 144 (2017) 520–535. [Google Scholar]
- [119].Kuo KC, Lin RZ, Tien HW, Wu PY, Li YC, Melero-Martin JM, Chen YC, Bioengineering vascularized tissue constructs using an injectable cell-laden enzymatically crosslinked collagen hydrogel derived from dermal extracellular matrix, Acta Biomater 27 (2015) 151–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Jusoh N, Oh S, Kim S, Kim J, Jeon NL, Microfluidic vascularized bone tissue model with hydroxyapatite-incorporated extracellular matrix, Lab Chip 15(20) (2015) 3984–8. [DOI] [PubMed] [Google Scholar]
- [121].Vishnu Priya M, Sivshanmugam A, Boccaccini AR, Goudouri OM, Sun W, Hwang N, Deepthi S, Nair SV, Jayakumar R, Injectable osteogenic and angiogenic nanocomposite hydrogels for irregular bone defects, Biomed Mater 11(3) (2016) 035017. [DOI] [PubMed] [Google Scholar]
- [122].Medici D, Shore E, Lounev V, Kaplan F, Kalluri R, Olsen B, Conversion of vascular endothelial cells into multipotent stem-like cells, Nature Medicine 16(12) (2010) 1400–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Cenni E, Perut F, Baldini N, In vitro models for the evaluation of angiogenic potential in bone engineering, Acta Pharmacol Sin 32(1) (2011) 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Zhu J, He P, Lin L, Jones DR, Marchant RE, Biomimetic poly(ethylene glycol)-based hydrogels as scaffolds for inducing endothelial adhesion and capillary-like network formation, Biomacromolecules 13(3) (2012) 706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Miller J, Shen C, Legant W, Baranski J, Blakely B, Chen C, Bioactive hydrogels made from step-growth derived PEG-peptide macromers, Biomaterials 31(13) (2010) 3736–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Sokic S, Christenson M, Larson J, Appel A, EM B, Papavasiliou G, Evaluation of MMP substrate concentration and specificity for neovascularization of hydrogel scaffolds, Biomaterials Science 2 (2014) 1343–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Turturro MV, Christenson MC, Larson JC, Young DA, Brey EM, Papavasiliou G, MMP-sensitive PEG diacrylate hydrogels with spatial variations in matrix properties stimulate directional vascular sprout formation, PLoS One 8(3) (2013) e58897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Hanjaya-Putra D, Wong KT, Hirotsu K, Khetan S, Burdick JA, Gerecht S, Spatial control of cell-mediated degradation to regulate vasculogenesis and angiogenesis in hyaluronan hydrogels, Biomaterials 33(26) (2012) 6123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kusuma S, Zhao S, Gerecht S, The extracellular matrix is a novel attribute of endothelial progenitors and of hypoxic mature endothelial cells, FASEB J 26(12) (2012) 4925–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Park KM, Gerecht S, Hypoxia-inducible hydrogels, Nat Commun 5 (2014) 4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Park KM, Blatchley MR, Gerecht S, The design of dextran-based hypoxia-inducible hydrogels via in situ oxygen-consuming reaction, Macromol Rapid Commun 35(22) (2014) 1968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Yao H, Liu N, Lin MC, Zheng J, Positive feedback loop between cancer stem cells and angiogenesis in hepatocellular carcinoma, Cancer Lett 379(2) (2016) 213–9. [DOI] [PubMed] [Google Scholar]
- [133].Cavaco A, Rezaei M, Niland S, Eble JA, Collateral Damage Intended-Cancer-Associated Fibroblasts and Vasculature Are Potential Targets in Cancer Therapy, Int J Mol Sci 18(11) (2017) 2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Dickinson LE, Lütgebaucks C, Lewis DM, Gerecht S, Patterning microscale extracellular matrices to study endothelial and cancer cell interactions in vitro, Lab Chip 12(21) (2012) 4244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Beckwith KM, Sikorski P, Patterned cell arrays and patterned co-cultures on polydopamine-modified poly(vinyl alcohol) hydrogels, Biofabrication 5(4) (2013) 045009. [DOI] [PubMed] [Google Scholar]
- [136].Lilu L, Zhaorong X, Wenyuan Z, Shimeng F, Jing K, Dong J, Jiao L, Xiaojie L, Xuesong Y, Yong L, Bingcheng L, Tingjiao L, Biomimetic tumor-induced angiogenesis and anti-angiogenic therapy in a microfluidic model, RSC Advances 6 (2016) 35248–35256 [Google Scholar]
- [137].Thakuri PS, Liu C, Luker GD, Tavana H, Biomaterials-Based Approaches to Tumor Spheroid and Organoid Modeling, Adv Healthc Mater 7(6) (2018) e1700980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Amann A, Zwierzina M, Koeck S, Gamerith G, Pechriggl E, Huber JM, Lorenz E, Kelm JM, Hilbe W, Zwierzina H, Kern J, Development of a 3D angiogenesis model to study tumour - endothelial cell interactions and the effects of anti-angiogenic drugs, Sci Rep 7(1) (2017) 2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Sobrino A, Phan DT, Datta R, Wang X, Hachey SJ, Romero-López M, Gratton E, Lee AP, George SC, Hughes CC, 3D microtumors in vitro supported by perfused vascular networks, Sci Rep 6 (2016) 31589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Nagaraju S, Truong D, Mouneimne G, Nikkhah M, Microfluidic Tumor-Vascular Model to Study Breast Cancer Cell Invasion and Intravasation, Adv Healthc Mater 7(9) (2018) e1701257. [DOI] [PubMed] [Google Scholar]
- [141].Yang S, Guo LJ, Microencapsulation of low-passage poorly-differentiated human mucoepidermoid carcinoma cells by alginate microcapsules: in vitro profiling of angiogenesis-related molecules, Cancer Cell Int 17 (2017) 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Zhang W, He X, Encapsulation of living cells in small ( approximately 100 microm) alginate microcapsules by electrostatic spraying: a parametric study, J Biomech Eng 131(7) (2009) 074515. [DOI] [PubMed] [Google Scholar]
- [143].Zhang H, Tumarkin E, Peerani R, Nie Z, Sullan RM, Walker GC, Kumacheva E, Microfluidic production of biopolymer microcapsules with controlled morphology, J Am Chem Soc 128(37) (2006) 12205–10. [DOI] [PubMed] [Google Scholar]
- [144].Kim C, Chung S, Kim YE, Lee KS, Lee SH, Oh KW, Kang JY, Generation of core-shell microcapsules with three-dimensional focusing device for efficient formation of cell spheroid, Lab Chip 11(2) (2011) 246–52. [DOI] [PubMed] [Google Scholar]
- [145].Agarwal P, Wang H, Sun M, Xu J, Zhao S, Liu Z, Gooch KJ, Zhao Y, Lu X, He X, Microfluidics Enabled Bottom-Up Engineering of 3D Vascularized Tumor for Drug Discovery, ACS Nano 11(7) (2017) 6691–6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Chan XY, Black R, Dickerman K, Federico J, Levesque M, Mumm J, Gerecht S, Three-Dimensional Vascular Network Assembly From Diabetic Patient-Derived Induced Pluripotent Stem Cells, Arterioscler Thromb Vasc Biol (2015) 2677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Zanotelli MR, Ardalani H, Zhang J, Hou Z, Nguyen EH, Swanson S, Nguyen BK, Bolin J, Elwell A, Bischel LL, Xie AW, Stewart R, Beebe DJ, Thomson JA, Schwartz MP, Murphy WL, Stable engineered vascular networks from human induced pluripotent stem cell-derived endothelial cells cultured in synthetic hydrogels, Acta Biomater 35 (2016) 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Belair DG, Schwartz MP, Knudsen T, Murphy WL, Human iPSC-derived endothelial cell sprouting assay in synthetic hydrogel arrays, Acta Biomater 39 (2016) 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Xiong YQ, Sun HC, Zhang W, Zhu XD, Zhuang PY, Zhang JB, Wang L, Wu WZ, Qin LX, Tang ZY, Human hepatocellular carcinoma tumor-derived endothelial cells manifest increased angiogenesis capability and drug resistance compared with normal endothelial cells, Clin Cancer Res 15(15) (2009) 4838–46. [DOI] [PubMed] [Google Scholar]
- [150].Franses JW, Drosu NC, Gibson WJ, Chitalia VC, Edelman ER, Dysfunctional endothelial cells directly stimulate cancer inflammation and metastasis, Int J Cancer 133(6) (2013) 1334–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Fuso Nerini I, Cesca M, Bizzaro F, Giavazzi R, Combination therapy in cancer: effects of angiogenesis inhibitors on drug pharmacokinetics and pharmacodynamics, Chin J Cancer 35(1) (2016) 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Diez M, Musri M, Ferrer E, Barbera J, Peinado V, Endothelial progenitor cells undergo an endothelial-to-mesenchymal transition-like process mediated by TGF beta RI, Cardiovascular Research 88(3) (2010) 502–511. [DOI] [PubMed] [Google Scholar]
- [153].Kitao A, Sato Y, Sawada-Kitamura S, Harada K, Sasaki M, Morikawa H, Shiomi S, Honda M, Matsui O, Nakanuma Y, Endothelial to Mesenchymal Transition via Transforming Growth Factor-beta 1/Smad Activation Is Associated with Portal Venous Stenosis in Idiopathic Portal Hypertension, American Journal of Pathology 175(2) (2009) 616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Cooley BC, Nevado J, Mellad J, Yang D, St Hilaire C, Negro A, Fang F, Chen G, San H, Walts AD, Schwartzbeck RL, Taylor B, Lanzer JD, Wragg A, Elagha A, Beltran LE, Berry C, Feil R, Virmani R, Ladich E, Kovacic JC, Boehm M, TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling, Sci Transl Med 6(227) (2014) 227ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Medici D, Potenta S, Kalluri R, Transforming growth factor-beta 2 promotes Snail-mediated endothelial-mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling, Biochemical Journal 437 (2011) 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Coll-Bonfill N, Musri MM, Ivo V, Barberà JA, Tura-Ceide O, Transdifferentiation of endothelial cells to smooth muscle cells play an important role in vascular remodelling, Am J Stem Cells 4(1) (2015) 13–21. [PMC free article] [PubMed] [Google Scholar]
- [157].Lin F, Wang N, Zhang TC, The role of endothelial-mesenchymal transition in development and pathological process, IUBMB Life 64(9) (2012) 717–23. [DOI] [PubMed] [Google Scholar]
- [158].Yamauchi K, Nishimura Y, Shigematsu S, Takeuchi Y, Nakamura J, Aizawa T, Hashizume K, Vascular endothelial cell growth factor attenuates actions of transforming growth factor-beta in human endothelial cells, J Biol Chem 279(53) (2004) 55104–8. [DOI] [PubMed] [Google Scholar]
- [159].Goumans MJ, Lebrin F, Valdimarsdottir G, Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways, Trends Cardiovasc Med 13(7) (2003) 301–7. [DOI] [PubMed] [Google Scholar]
- [160].Munger J, Harpel J, Giancotti F, Rifkin D, Interactions between growth factors and integrins: Latent forms of transforming growth factor-beta are ligands for the integrin alpha v beta 1, Molecular Biology of the Cell 9(9) (1998) 2627–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Alghisi G, Ponsonnet L, Ruegg C, The Integrin Antagonist Cilengitide Activates alpha V beta 3, Disrupts VE-Cadherin Localization at Cell Junctions and Enhances Permeability in Endothelial Cells, Plos One 4(2) (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Liebner S, Cattelino A, Gallini R, Rudini N, Iurlaro M, Piccolo S, Dejana E, beta-Catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse, Journal of Cell Biology 166(3) (2004) 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Aisagbonhi O, Rai M, Ryzhov S, Atria N, Feoktistov I, Hatzopoulos A, Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition, Disease Models & Mechanisms 4(4) (2011) 469–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Zhang Z, Wang J, Xu Y, Jiang Z, Wu R, Wang L, Chen P, Hu X, Yu H, Menstrual blood derived mesenchymal cells ameliorate cardiac fibrosis via inhibition of endothelial to mesenchymal transition in myocardial infarction, International Journal of Cardiology 168(2) (2013) 1711–1714. [DOI] [PubMed] [Google Scholar]
- [165].Xu X, Tan X, Hulshoff MS, Wilhelmi T, Zeisberg M, Zeisberg EM, Hypoxia-induced endothelial-mesenchymal transition is associated with RASAL1 promoter hypermethylation in human coronary endothelial cells, FEBS Lett 590(8) (2016) 1222–33. [DOI] [PubMed] [Google Scholar]
- [166].Liu L, Chen J, Sun L, Xu Y, RhoJ promotes hypoxia induced endothelial-to-mesenchymal transition by activating WDR5 expression, J Cell Biochem 119(4) (2018) 3384–3393. [DOI] [PubMed] [Google Scholar]
- [167].Xu X, Tan X, Tampe B, Sanchez E, Zeisberg M, Zeisberg EM, Snail Is a Direct Target of Hypoxia-inducible Factor 1α (HIF1α) in Hypoxia-induced Endothelial to Mesenchymal Transition of Human Coronary Endothelial Cells, J Biol Chem 290(27) (2015) 16653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Liu Y, Zou J, Li B, Wang Y, Wang D, Hao Y, Ke X, Li X, RUNX3 modulates hypoxia-induced endothelial-to-mesenchymal transition of human cardiac microvascular endothelial cells, Int J Mol Med 40(1) (2017) 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Choi SH, Hong ZY, Nam JK, Lee HJ, Jang J, Yoo RJ, Lee YJ, Lee CY, Kim KH, Park S, Ji YH, Lee YS, Cho J, A Hypoxia-Induced Vascular Endothelial-to-Mesenchymal Transition in Development of Radiation-Induced Pulmonary Fibrosis, Clin Cancer Res 21(16) (2015) 3716–26. [DOI] [PubMed] [Google Scholar]
- [170].Mahler GJ, Frendl CM, Cao Q, Butcher JT, Effects of shear stress pattern and magnitude on mesenchymal transformation and invasion of aortic valve endothelial cells, Biotechnol Bioeng 111(11) (2014) 2326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Zhang H, Chang H, Wang LM, Ren KF, Martins MC, Barbosa MA, Ji J, Effect of Polyelectrolyte Film Stiffness on Endothelial Cells During Endothelial-to-Mesenchymal Transition, Biomacromolecules 16(11) (2015) 3584–93. [DOI] [PubMed] [Google Scholar]
- [172].Zeng ZZ, Yao H, Staszewski ED, Rockwood KF, Markwart SM, Fay KS, Spalding AC, Livant DL, alpha(5)beta(1) Integrin Ligand PHSRN Induces Invasion and alpha(5) mRNA in Endothelial Cells to Stimulate Angiogenesis, Transl Oncol 2(1) (2009) 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Patel ZS, Mikos AG, Angiogenesis with biomaterial-based drug- and cell-delivery systems, J Biomater Sci Polym Ed 15(6) (2004) 701–26. [DOI] [PubMed] [Google Scholar]
- [174].Bhise NS, Shmueli RB, Sunshine JC, Tzeng SY, Green JJ, Drug delivery strategies for therapeutic angiogenesis and antiangiogenesis, Expert Opin Drug Deliv 8(4) (2011) 485–504. [DOI] [PMC free article] [PubMed] [Google Scholar]