Figure 1.
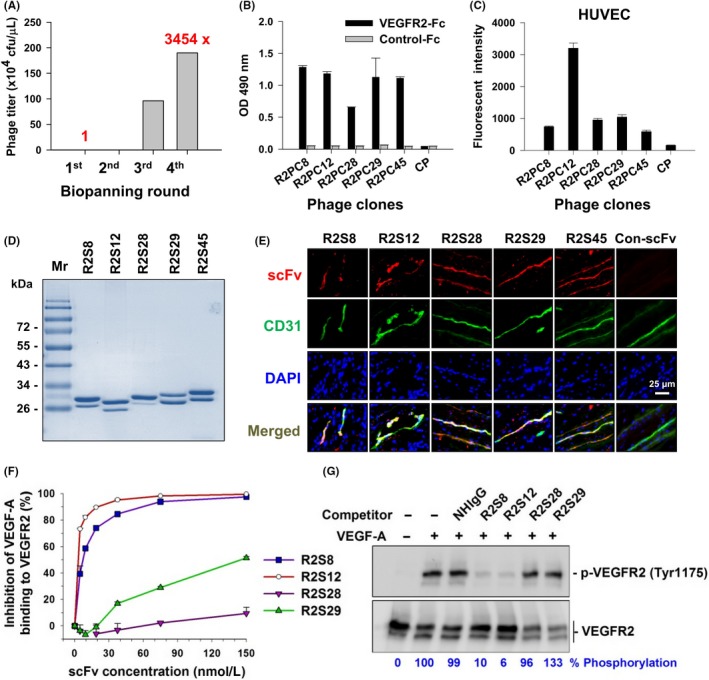
Selection and identification of vascular endothelial growth factor receptor‐2 (VEGFR2)‐binding single chain fragment variables (scFvs). A, After 4 rounds of biopanning for VEGFR2‐Fc recombinant protein, the phage recovery rate was increased by 3454‐fold over that of the first round. cfu, colony‐forming units. B, Comparison of the binding of selected phage clones to VEGFR2‐Fc protein by ELISA with a 1 × 109 cfu phage titer. CP, control phage used as a negative control; OD, optical density. C, Binding affinity of phage clones to cellular VEGFR2 was evaluated with flow cytometry of HUVECs and 1 × 1010 cfu phage titer. D, Soluble anti‐VEGFR2 scFvs were purified and analyzed by SDS‐PAGE with Coomassie blue staining. Mr, molecular weight. E, Immunofluorescence for human tumor vasculature. Frozen sections of surgical specimens of lung cancer patients were probed with anti‐VEGFR2 scFvs, followed by anti‐E tag Ab and rhodamine‐conjugated secondary Ab. Vascular endothelium was stained with anti‐human CD31 Ab and FITC‐conjugated secondary Ab. Nuclei were stained with DAPI. Con‐scFv, control scFv. F, Competitive binding of anti‐VEGFR2 scFv and vascular endothelial growth factor‐A (VEGF‐A) was analyzed by ELISA. VEGF‐A binding to immobilized VEGFR2 in the absence of competitors was considered to be 100%. G, Phosphorylated VEGFR2 (p‐VEGFR2) expression in HUVECs treated with VEGF‐A and scFv competitors was detected by western blot. Quantification of p‐VEGFR2 was based on luminescence intensity and normalized to total VEGFR2. NHIgG, normal human IgG. Error bars, SE
