Figure 2.
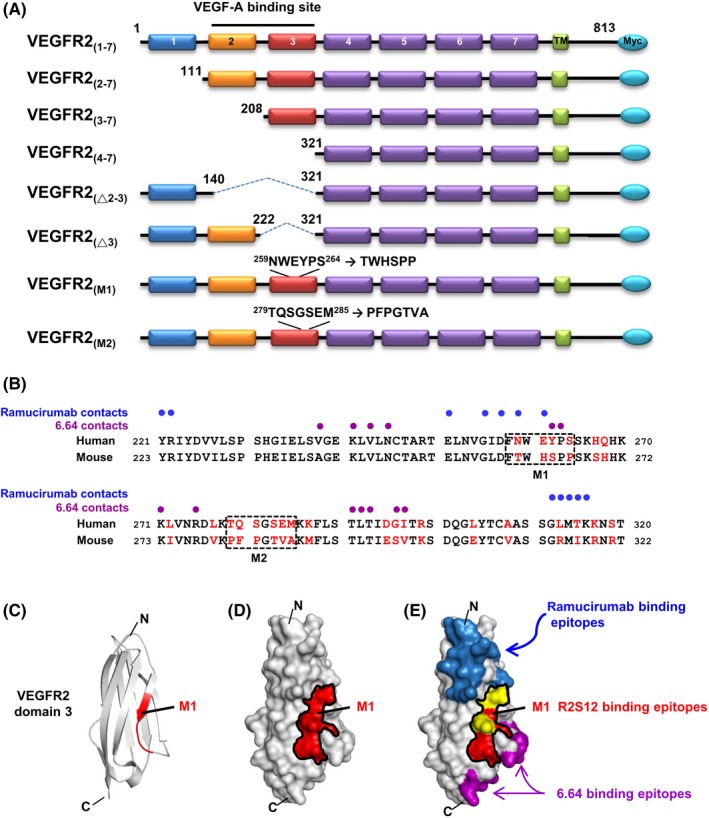
B‐cell epitope mapping of antivascular endothelial growth factor receptor‐2 (VEGFR2) single chain fragment variable R2S12. A, Schematic presentation of the human VEGFR2 domains and its deletion or substitution mutants used in this study is shown. There are 7 Ig‐like domains in the extracellular region of VEGFR2, labeled 1‐7. TM, transmembrane domain. B, Sequence alignment of human and mouse VEGFR2 domain 3. Residues that differ between the 2 species are highlighted in red. M1 and M2 clusters are marked with dashed‐line boxes. Blue and purple dots indicate human VEGFR2 domain 3 residues in contact with ramucirumab and 6.64 Abs, respectively. C, Ribbon diagram for the human VEGFR2 domain 3 structure; NWEYPS residues (M1) responsible for R2S12 binding are highlighted in red. D, Model of the surface of VEGFR2 domain 3. NWEYPS residues are indicated in red, and the M1 area is delineated by a black line. E, Residues that make contact with ramucirumab and 6.64 on the surface of VEGFR2 domain 3 are shown in blue and purple, respectively. The contacting residues for ramucirumab that are localized in the M1 area are shown in yellow. C, C terminus; N, N terminus
