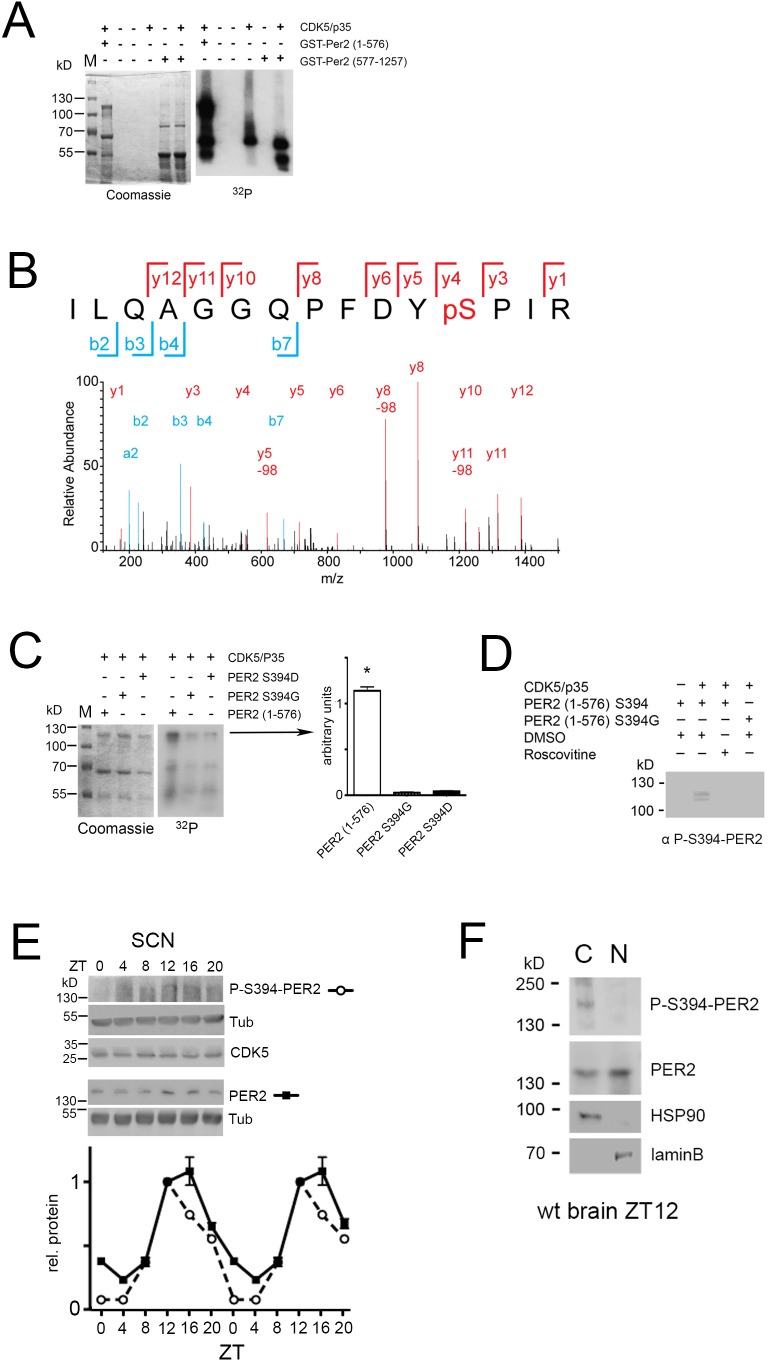
Figure 5. CDK5 phosphorylates PER2 at S394.
(A) An in vitro kinase assay was performed using recombinant CDK5/p35 and either GST-PER2 1–576 or GST-PER2 577–1256 as substrate. The samples were subjected to 10% SDS page (Coomassie, left panel) and the phosphorylation of PER2 was detected by autoradiography in order to visualize 32P-labeled proteins (right panel). CDK5 phosphorylates the N-terminal half (1-576) of a GST-PER2 fusion protein whereas the C-terminal half (577–1257) is not phosphorylated. The signal for CDK5/p35 alone indicates CDK5 auto-phosphorylation seen in all lanes when CDK5 is present. (B) Annotated mass spectrum of the tryptic peptide PER2383-397 ILQAGGQPFDYpSPIR containing the phosphorylated residue S394. The red color depicts the y-ion series (1-12) and blue the b-ion series (2–7, a2); y5-98, y8-98, y11-98 show the de-phosphorylated ions. (C) In vitro kinase assay was performed as in (A). The putative phosphorylation site was mutated to aspartic acid (S394D) or glycine (S394G). Both mutations abrogated the CDK5-mediated phosphorylation. Coomassie staining reveals equal expression of the GST-PER2 fragments. The bar diagram at the right shows the quantification of three experiments. One-way-ANOVA with Bonferroni’s post-test, *: p<0.001 (D) The monoclonal antibody produced against P-S394-PER2 does recognizes PER2 (1–576) S394 phosphorylation mediated by CDK5/p35 in presence but not in absence of the kinase or when CDK5 is inactivated by roscovitine. This antibody does not recognize the S394G mutated form even in presence of CDK5/p35. (E) Temporal profile of P-S394-PER2 and total PER2 in SCN tissue. Upper panels show western blots of the corresponding proteins indicated on the right. Below the quantification of three experiments is shown, in which the value at ZT12 of PER2 has been set to 1. The data were double plotted. Values are the mean ± SEM. Two-way ANOVA with Bonferroni’s multiple comparisons revealed that the two curves are significantly different with p<0.0001, F = 93.65, DFn = 1, DFd = 48. (F) Subcellular localization of P-S394-PER2. Total wild-type mouse brain extracts were separated into cytoplasmic (HSP90 positive) and nuclear (laminB positive) fractions. Phosphorylated PER2 was predominantly detected in the cytoplasm with the P-S394-PER2 antibody, whereas the general PER2 antibody detected PER2 in both compartments with higher amounts in the nuclear fraction.
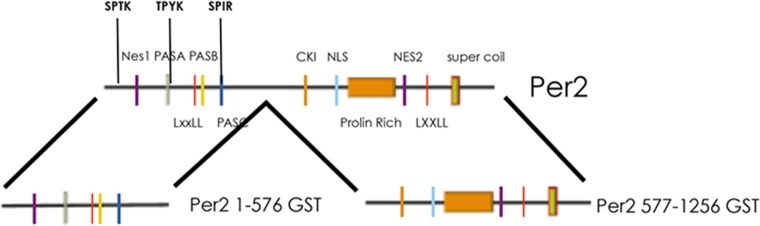
Figure 5—figure supplement 1. Scheme of PER2 fragments used for the in vitro kinase assay.
Figure 5—figure supplement 2. Additional controls for in vitro kinase assay.
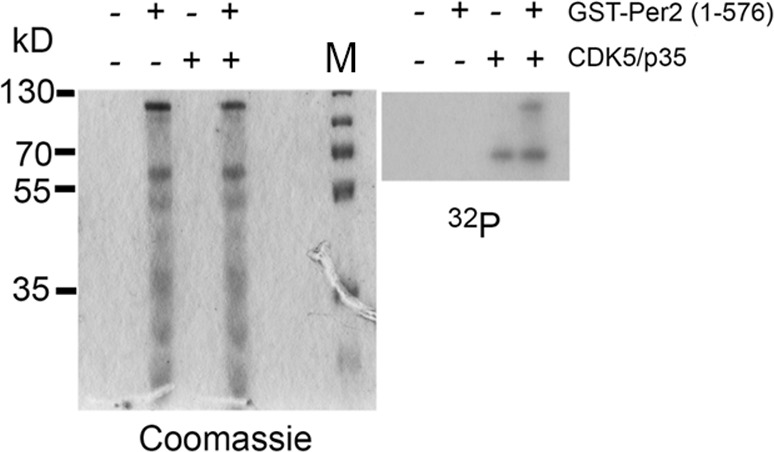
Figure 5—figure supplement 3. Testing specificity of the in vitro kinase assay.
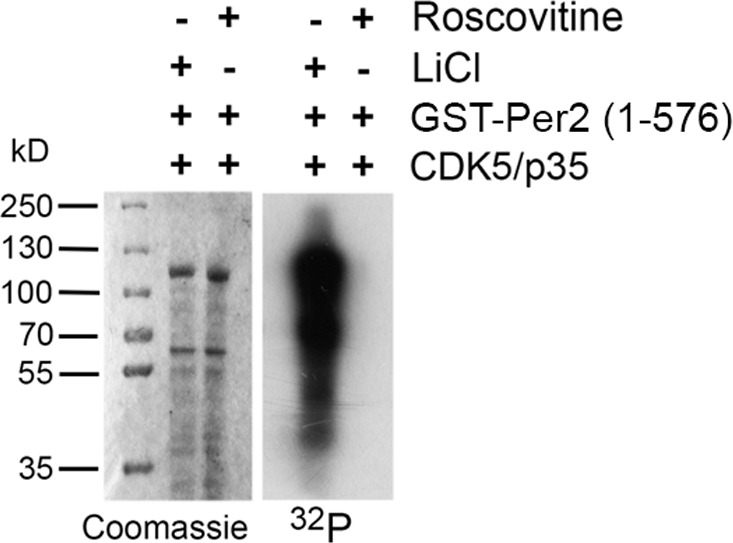
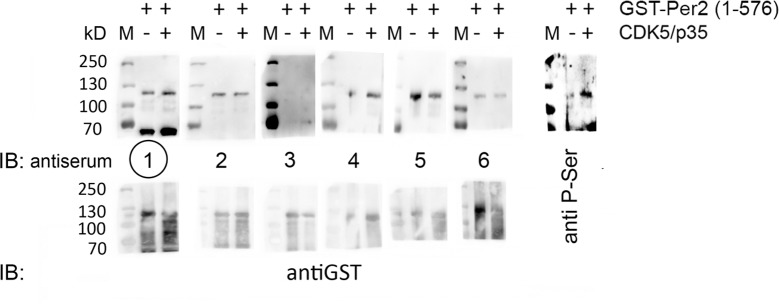
Figure 5—figure supplement 4. Characterization of antisera against P-S394-PER2.
Figure 5—figure supplement 5. Characterization of hybridomas against P-S394-PER2.
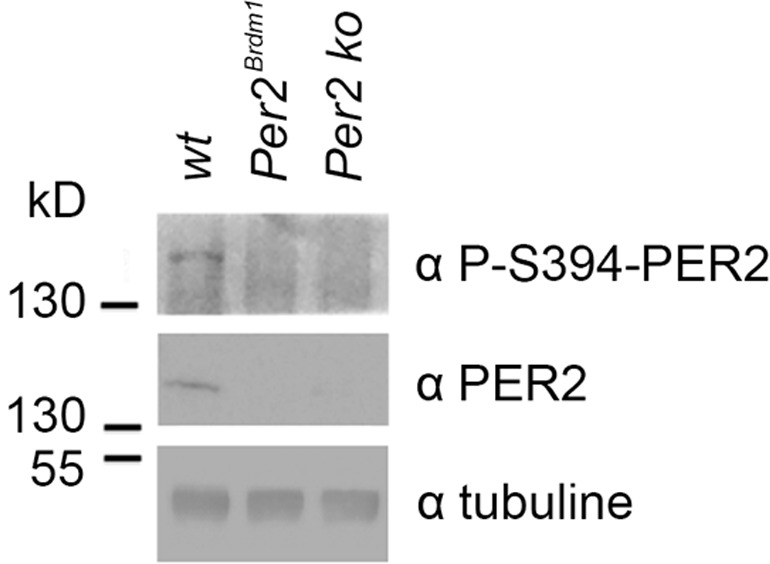
Figure 5—figure supplement 6. Validation of anti-P-S394-PER2 antibody.