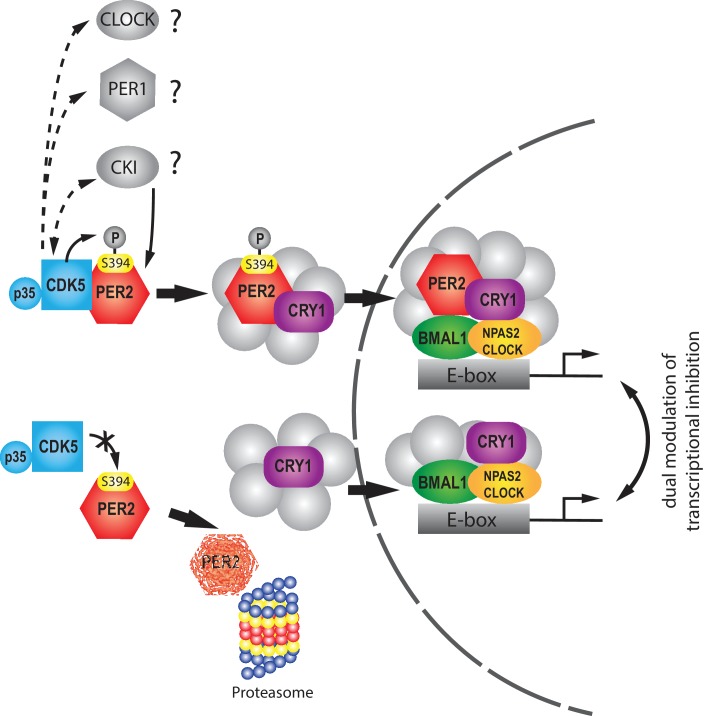
Figure 7. Model illustrating the regulation of PER2 by CDK5.
The upper row illustrates phosphorylation of PER2 at S394 by CDK5 that subsequently favors interaction with CRY1 and leads to transport into the nucleus, where the PER2/CRY1 complex inhibits BMAL1/NPAS2 (or in the periphery CLOCK)-driven transcriptional activation. Of note is that CDK5 potentially phosphorylates other clock relevant components, such as CLOCK, PER1 and CKI. The lower part illustrates that inhibition of CDK5 leads to a lack of S394 PER2 phosphorylation, which renders the PER2 protein more prone to degradation by the proteasome. CRY1 does not form a complex with PER2 and hence PER2 is not transported into the nucleus. CRY1 enters the nucleus independently and can inhibit the BMAL1:NPAS2 (or in the periphery CLOCK) transcriptional complex. This model is consistent with the dual modulation of transcriptional inhibition (Ye et al., 2014; Xu et al., 2015). Transcriptional inhibition is modulated in an intricate unknown manner by various additional factors (gray) (Aryal et al., 2017) that may be cell type specific.