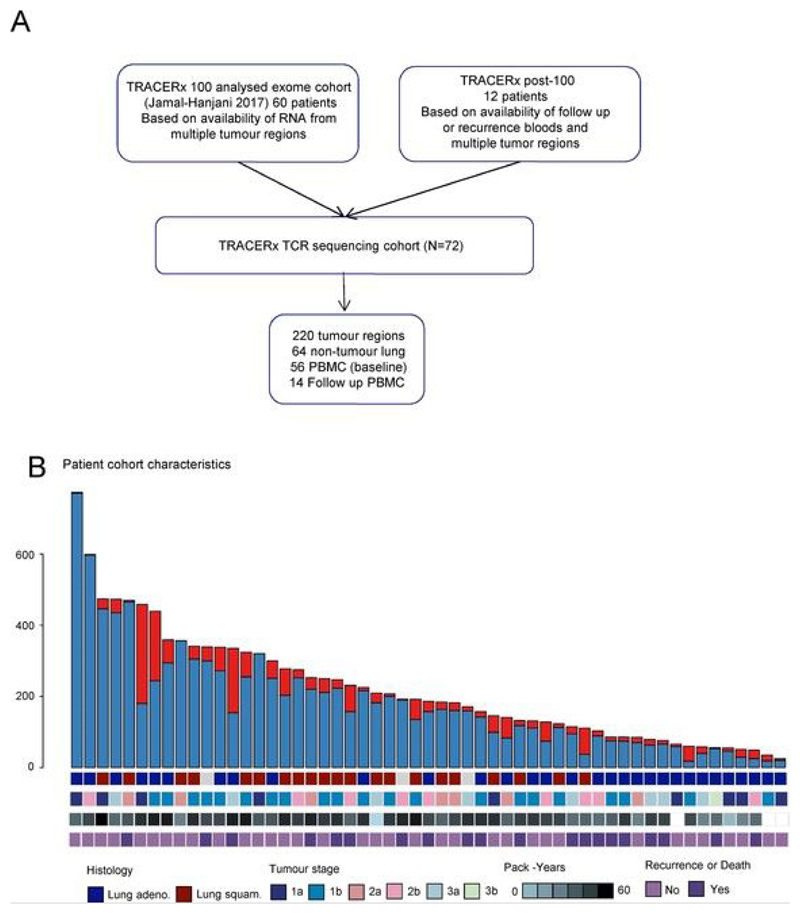
Extended Data Fig. 1. Patient selection, mutational burden and clinical characteristics.
a, CONSORT diagram showing the selection of TRACERx patients for TCR sequencing. b, The total number of nonsynonymous mutations (clonal and subclonal) and patient clinical characteristics (histology, stage, smoking status and clinical outcome) for the TCR sequencing cohort are shown.