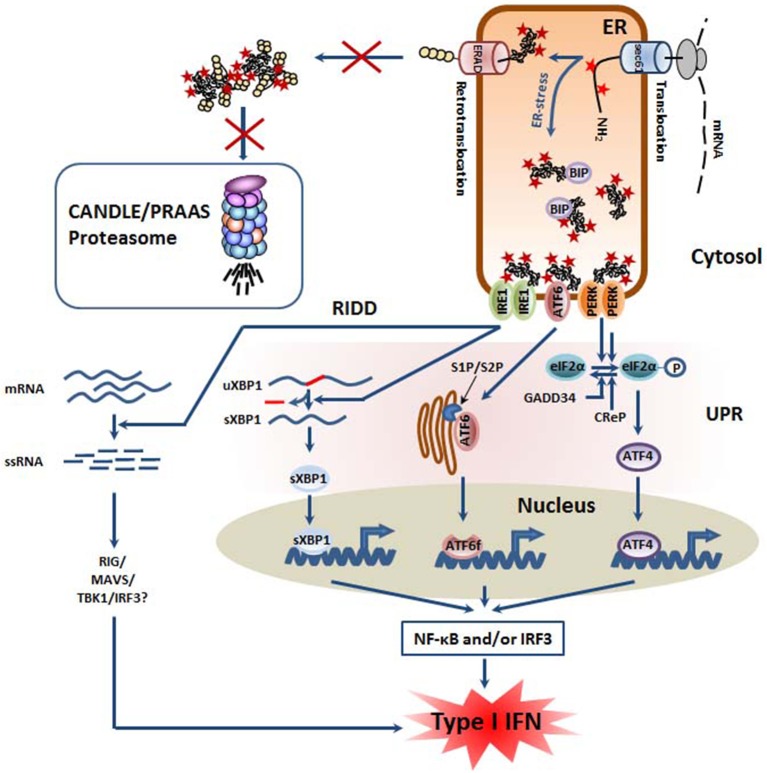
Figure 2.
Defective proteasomes in CANDLE/PRAAS subjects provoke ER stress and trigger the so-called unfolded protein response (UPR) which is associated with inflammation. Defective proteasomes impair the ER-associated degradation (ERAD) of misfolded ER proteins, leading to their accumulation within the lumen. Perturbed protein homeostasis is then sensed by the three ER membrane-resident proteins IRE1, ATF6, and PERK which initiate a complex signaling program known as the unfolded protein response (UPR). Thanks to its endonuclease activity, IRE1 promotes the splicing of the untranslated XBP1 mRNA, thus giving rise to spliced XBP1 mRNA species encoding an active transcription factor. The ATF6 protein is transported into the Golgi apparatus where it is subjected to a proteolytic cleavage by the site-1 and−2 proteases (S1P and S2P), thereby generating an ATF6f transcription factor. By promoting the phosphorylation of eIF2α, PERK favors the cap-independent translation of stress proteins such as the ATF4 transcription factor. All transcription factors (sXBP1, ATF6f, and ATF4) activated by the UPR have been shown to initiate sterile inflammation by favoring the activation of the NF-κB and/or IRF3 transcription factors. In addition, IRE1 is implicated in the regulated IRE1-dependent decay (RIDD) pathway in which cellular mRNA are subjected to degradation, thus resulting in the generation of 5′ and 3′ unprotected single stranded RNA which may be sensed as foreign RNA and induce a type I IFN response following their recognition by pathogen recognition receptors (PRR) including RIG-I.