Figure 4.
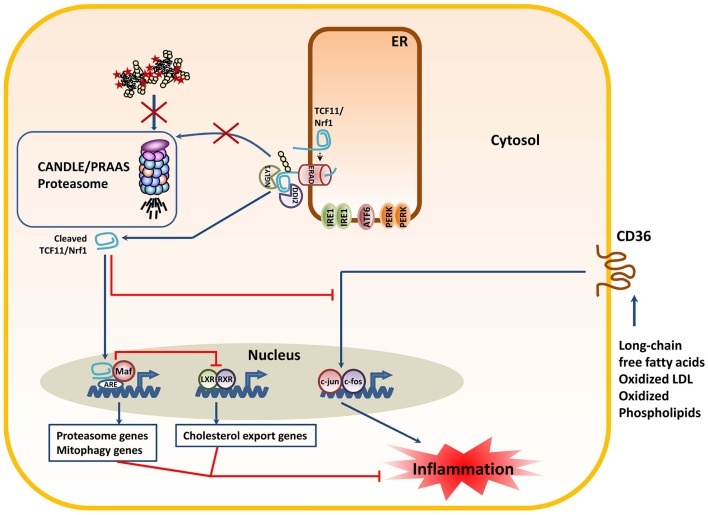
The decreased proteasome activity in subjects with CANDLE/PRAAS triggers the processing of the TCF11/Nrf1 transcription factor with anti-inflammatory consequences. Proteasome dysfunction due to loss-of-function mutations prevents the rapid degradation of the TCF11/Nrf1 protein by ERAD, thus resulting in its de-glycosylation by NGLY1 and subsequent proteolytic cleavage by DDI2. The C-terminal TCF11/Nrf1 processed fragment enters the nucleus to form heterodimers with small Maf proteins and induce the transcription of proteasome and mitophagy genes. In addition, processed TCF11/Nrf1 promotes the trans-respression of LXR-responsive genes such as those involves in cholesterol export. Both of these events are considered anti-inflammatory, as they prevent the harmful leakage of mitochondrial DNA into the cytosol and preserve cholesterol homeostasis in the cell. The anti-inflammatory properties of TCF11/Nrf1 are further exemplified by its capacity to suppress the signaling cascade triggered by the CD36 upon binding to pro-inflammatory lipid molecules.