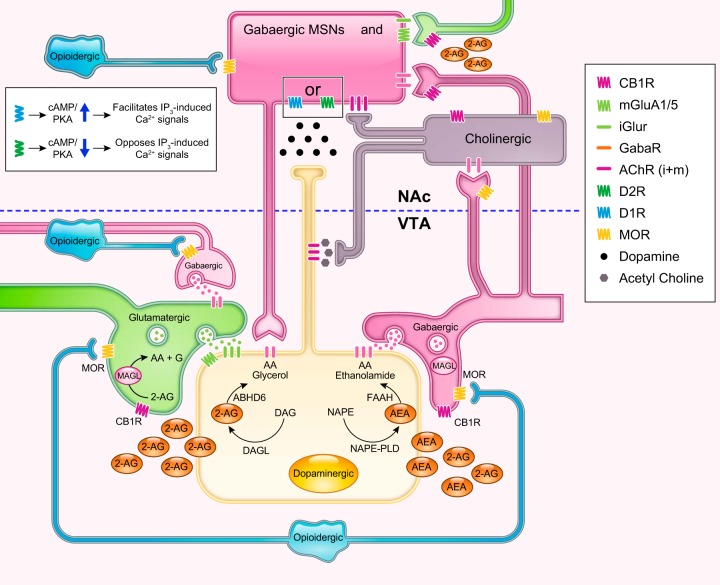
FIGURE 2.
Schematic simplified cartoon showing some of the indirect modulatory effects of midbrain (ventral tegmental area, VTA) opioid and endocannabinoid signals on dopaminergic transmission in nucleus accumbens (NAc). Reward-related stimuli conveyed through glutamatergic afferents (green) promote burst firing of dopamine (DA) neurons (yellow) mainly driven by ionotropic glutamate receptor (iGluR) binding activation at the dopaminergic cell. The level of activation is normally kept in check by GABAergic counterbalancing inputs (pink), but also by direct inhibitory GABAergic input inhibiting presynaptic glutamate release (66). Endogenous [released from opioidergic neurons (light blue), mostly projecting from the hypothalamus] or exogenous (natural or synthetic opioid like molecules) opioids activate endogenous mu opioid receptors (MOR) on GABAergic interneurons. The MOR is coupled to inhibitory G proteins, whose activation (by an endogenous peptide like endorphin or exogenous agonists like morphine and fentanyl) leads to a dissociation between the Gα and Gβγ subunits and the activation of intracellular effector pathways. One such pathway leads to the inhibition of GABA release as a result of increased conduction of potassium ions, which hyperpolarizes the cell making it less responsive to depolarizing inputs and inhibiting calcium influx (123). In addition, activation of MOR increases mitogen-activated protein kinase (MAPK) signaling while their phosphorylation activates the arrestin pathway (5), which has the ability to desensitize, activate, and control the trafficking of G protein-coupled receptors (GPCR) (140). A drop in GABAergic tone causes a net disinhibition of the neighboring dopaminergic neuron and the release of excess dopamine (black dots) onto direct and indirect medium spiny neurons [pink medium spiny neuron (MSN)], which reinforces the euphorigenic effects of opioids. Ionotropic GluR-mediated activation of the DA neuron leads to Ca2+ influx (via voltage-gated calcium channels), which is either facilitated or hampered in D1R vs D2R expressing MSN populations, respectively (317) (inset), leading to their differential roles in plasticity. At the same time, the Ca2+ influx, combined with activation of mGluA1/5, triggers the “on demand” production of 2-arachidonoylglycerol (2-AG) from diacylglycerol (DAG) [or anandamide (AEA) from N-acyl-phosphatidylethanolamines (NAPE)]. Retrograde 2-AG transmission through CB1 receptor binding on monoacylglycerol lipase (MAGL) containing afferent (GABA and Glu) neurons has the net effect of disinhibiting dopamine neurons and facilitating phasic DA release (63). This is because cannabinoids (e.g., tetrahydrocannabinol, 2-AG) operate as full agonists at GABA terminals [that display a high CB1R to vesicles ratio (188)] but as partial agonists at Glu terminals [where the CB1R-to-vesicles ratio is much lower (295, 296)]. As shown, AEA is assumed to be retrograde in spite of data showing that FAAH is predominately postsynaptic while NAPE PLD is presynaptic. The true nature of AEA neurotransmission remains unclear partly because there are other pathways for AEA synthesis. In the NAc, GABAergic projections, sent by the VTA, also synapse onto cholinergic interneurons (dark gray), thus inhibiting their excitatory input onto DA terminals. Activation of either CB1 or MOR on these GABA neurons can stimulate DA terminals (independently of VTA DA neuron activation) by disinhibiting ACh release while activation of these receptors, which are also expressed on ACh interneurons, could in theory have the opposite effect on DA levels in the accumbens (351). GABAergic and glutamatergic terminals in the NAc also have the capacity to modulate accumbal DA activity onto MSNs directly. Since these neurons also express MOR [but some also CB1R (226, 361)], their activation on GABA inputs could enhance DA release, while their inhibitory effects on glutamatergic inputs could reduce accumbal release of DA.