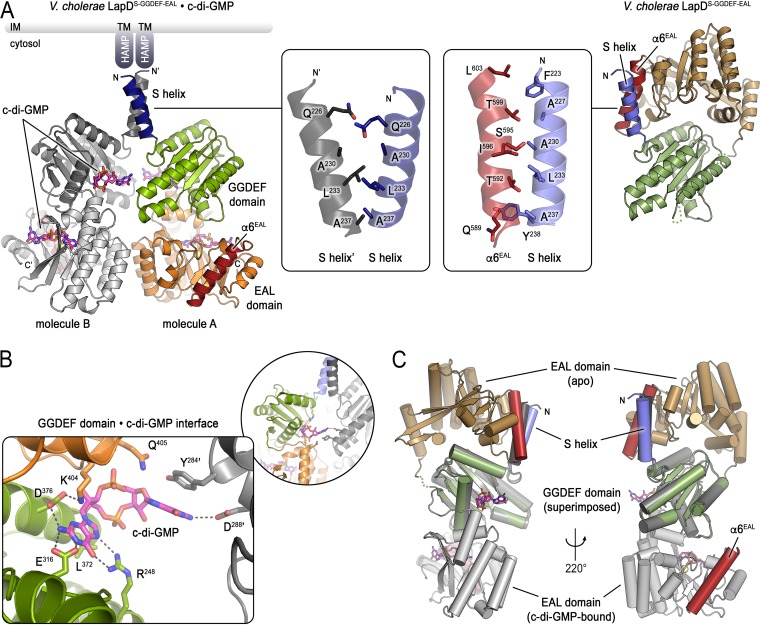
FIG 2.
Structure of the c-di-GMP-bound cytoplasmic module of V. cholerae LapD. (A) Comparison between the c-di-GMP-bound and unbound structures of V. cholerae LapD’s cytoplasmic module. The c-di-GMP-bound protein crystallized with two protein molecules per asymmetric unit (left) (molecule A: S helix, dark blue; GGDEF domain, light green; EAL domain, orange with red α6 helix; molecule B: shades of gray). GGDEF and EAL domains bind a single c-di-GMP molecule each. The insets show the S helix dimerization interface in the c-di-GMP-bound dimer (left inset) or the S helix-α6 helix (EAL domain) interface of the apo state (right inset). The apo state is shown for comparison in a similar view as the in the left panel with regard to the S helix-GGDEF domain fragment (right) (S helix, slate; GGDEF domain, dark green; EAL domain, tan with red α6 helix). LapD’s periplasmic domains are not shown. (B) Detailed view of the c-di-GMP binding site on the GGDEF domain of V. cholerae LapD (colored as in panel A, left). (C) Superposition the structures of apo- and c-di-GMP-bound V. cholerae LapD. The respective GGDEF domains were superimposed to highlight the conformational change of this regulatory module. The relative position of the S helix would be fixed by the preceding HAMP and transmembrane domains (not shown). Two views separated by a 220° rotation along the y axis are shown. The apo state and c-di-GMP-bound (molecule B) protomers are colored as in panel A.