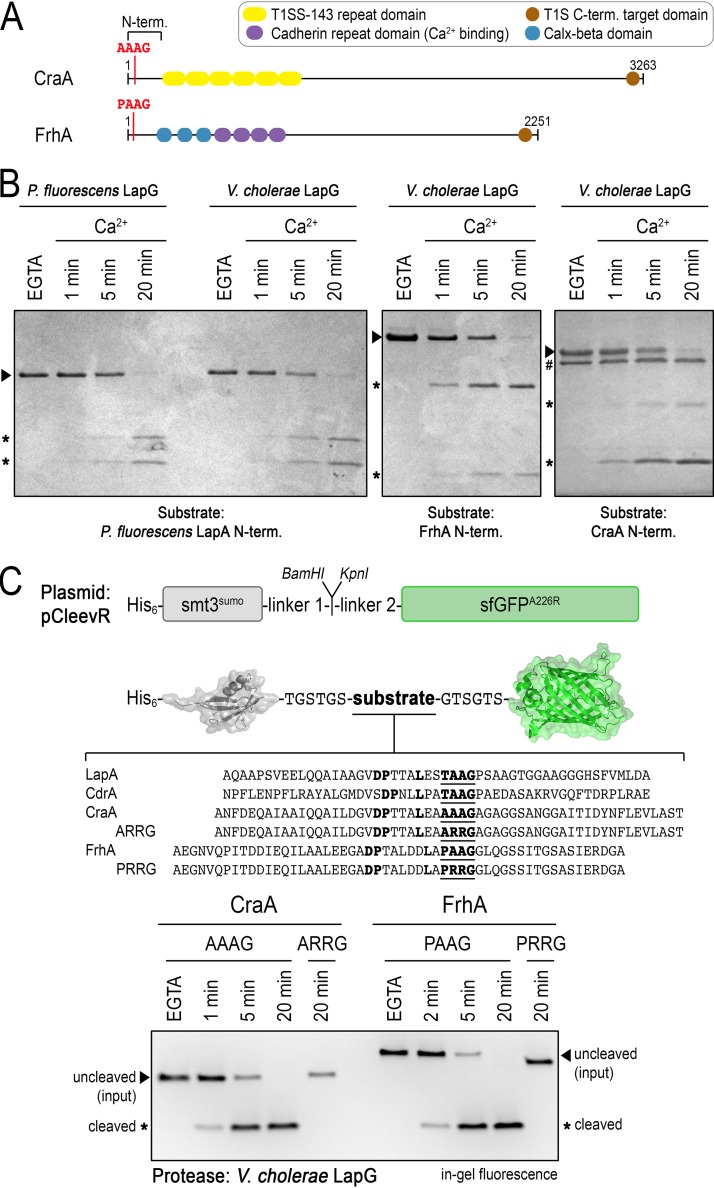
FIG 4.
V. cholerae LapG is an active calcium-dependent protease. (A) Domain organization of CraA and FrhA. The N-terminal domain comprises the periplasmic retention module and predicted consensus sequence for cleavage by LapG. (B) V. cholerae LapG proteolyzes the N-terminal fragments of P. fluorescens LapA, FrhA, and CraA in a calcium-dependent manner. Processing of CraA (relative to LapA and FrhA) required 10-fold more LapG (0.5 versus 5 μM LapG; LapG is indicated by # in the gel showing CraA processing). Representative gels from three independent experiments are shown. (C) Characterization of LapG specificity with the pCleevR assay. A diagram is shown of the pCleevR constructs in which various adhesin sequences containing their respective LapG cleavage sites are flanked N terminally by a His6-tagged SUMO protein and C terminally by sfGFP-A226R. The SDS-PAGE, imaged by in-gel fluorescence to detect fragments containing the sfGFP module, shows LapG activity toward peptides from CraA and FrhA. Proteolysis was prevented in the presence of EGTA or when the consensus di-alanine motif, the site where LapG cleaves its substrates, was mutated to a di-arginine motif. Representative gels from three independent experiments are shown.