Short abstract
Background
Patient-reported outcomes (PRO) and clinical outcomes give a broad assessment of relapsing–remitting multiple sclerosis (RRMS) disease.
Objective
The aim is to evaluate the effectiveness of delayed-release dimethyl fumarate (DMF) on disease activity and PROs in patients with RRMS in the clinic.
Methods
PROTEC, a phase 4, open-label, 12-month observational study, assessed annualized relapse rate (ARR), proportion of patients relapsed, and changes in PROs. Newly diagnosed and early MS (≤3.5 EDSS and ≤1 relapse in the prior year) patient subgroups were evaluated.
Results
Unadjusted ARR at 12 months post-DMF versus 12 months before DMF initiation was 75% lower (0.161 vs. 0.643, p < 0.0001) overall (n = 1105) and 84%, 77%, and 71% lower in newly diagnosed, ≤3.5 EDSS, and ≤1 relapse subgroups, respectively. Overall, 88% of patients were relapse-free 12 months after DMF initiation (84%, newly diagnosed; 88%, ≤3.5 EDSS; 88%, ≤1 relapse). PRO measures for fatigue, treatment satisfaction, daily living, and work improved significantly over 12 months of DMF versus baseline.
Conclusion
At 12 months after versus 12 months before DMF initiation, ARR was significantly lower, the majority of patients were relapse-free, and multiple PRO measures showed improvement (overall and for subgroups), suggesting that DMF is effective based on clinical outcomes and from a patient perspective.
Clinical trial: A Study Evaluating the Effectiveness of Tecfidera (Dimethyl Fumarate) on Multiple Sclerosis (MS) Disease Activity and Patient-Reported Outcomes (PROTEC), NCT01930708, https://clinicaltrials.gov/ct2/show/NCT01930708.
Keywords: Dimethyl fumarate, multiple sclerosis, relapsing–remitting, patient-reported outcome measures, quality of life, treatment outcomes, activities of daily living
Introduction
The debilitating character of multiple sclerosis (MS) makes the assessment of quality of life (QoL) and patient-reported outcomes (PROs) very important to the patient, their families, and healthcare providers. PROs are valuable tools for assessing disease states and the effects of treatments from the patients’ perspective, allowing direct feedback on their well-being and the impact of treatment on their health. PROs can provide a broader overall assessment of MS disease state and progression than clinical and neurological outcome measures such as annualized relapse rate (ARR).1,2
Delayed-release dimethyl fumarate (DMF) is approved as an oral treatment for relapsing and relapsing–remitting MS (RRMS).3–5 As of 30 June 2019, >415,000 patients have been treated with DMF, representing >780,000 patient-years of exposure; ∼6335 patients (14,065 patient-years) were from clinical trials (Biogen data on file). DMF demonstrated sustained efficacy on clinical and neuroradiological measures, and a favorable benefit-risk profile, in patients with RRMS in two phase 3 studies, DEFINE (NCT00420212) and CONFIRM (NCT00451451), and a phase 4 long-term extension study ENDORSE (NCT00835770).6–8 PROs were significantly improved with DMF treatment versus placebo between integrated analysis of DEFINE and CONFIRM, measured by EuroQol 5-Dimensions visual analogue scale (EQ-5D VAS) and 36-Item Short Form Health Survey,9 not measured in a clinical setting. Patients with RRMS receiving DMF experienced increased work productivity and health-related QoL (HRQoL), measured by the EuroQol-5D (EQ-5D), versus patients receiving beta interferons or glatiramer acetate.10
Patients with RRMS have better long-term outcomes when treated early with therapy controlling disease activity.11 In post hoc analyses, efficacy in patients with early MS, including newly diagnosed patients, was consistent with the overall study populations.8,12
This analysis presents the primary outcome results from PROTEC (NCT01930708), a 12-month observational study that evaluated DMF’s effectiveness on disease activity and PROs in patients with RRMS in a real-world clinical setting. An exploratory endpoint subgroup analysis evaluated DMF’s effectiveness in newly diagnosed and other patients with early MS in PROTEC.
Materials and methods
Study design
PROTEC was a phase 4, open-label, single-arm, observational, multicenter study conducted in Europe and Canada from October 2013 to March 2016. Patients received the approved dosage of DMF orally, 120 mg twice daily during the first week and 240 mg twice daily thereafter. Relapses, Expanded Disability Status Scale (EDSS), and adverse events (AEs) were evaluated at scheduled and unscheduled visits. PROs were assessed at clinic visits scheduled at Baseline, 3, 6, and 12 months after DMF treatment initiation. The EDSS scores13 ranged from 0.0 (normal) to 10.0 (death due to MS). Patients were assessed for relapses over the 12 months prior to DMF initiation.
Patients
Patient eligibility criteria included: age ≥18 years, diagnosis of RRMS, and no prior treatment with DMF or second-line MS therapies (e.g. natalizumab, fingolimod, alemtuzumab). Exclusion criteria included pregnancy or risk of pregnancy (unless approved by Investigator), currently receiving other RRMS therapies (e.g. interferon-β, glatiramer acetate, or teriflunomide), hypersensitivity to DMF active component, or current enrollment in other clinical trials, other than Pregnancy Exposure Registry or other non-conflicting studies. Patients discontinued DMF if their lymphocyte counts were < 0.5 × 109/l for 24 weeks.
Primary and secondary endpoints
The primary endpoint reported ARR 12 months after DMF treatment in patients with RRMS. The secondary endpoints evaluated mean changes in PRO scores from Baseline over 12 months; proportion of patients with confirmed 24-week EDSS progression at 12 months after enrollment; ARR over 12 months before enrollment and at 12 months after enrollment; proportion of patients relapsed at 12 months after enrollment; proportion of patients with relapses associated with intravenous steroid use; and proportion of patients who report taking the prescribed DMF dose, overall percent adherence, and reasons for not taking full dose over a 12-month period. Additional outcomes included association between health care resource utilization and disease activity with PROs over a 12-month period, and association between patient-reported adherence and PROs over a 12-month period.
Patient-reported outcomes
For assessment of DMF impact on PROs, patients completed the following instruments (language translated). The PRIMUS-Activity Limitations questionnaire was not completed in Portugal due to lack of available local translation. The Multiple Sclerosis Impact Scale (MSIS-29) measures the physical (20 items) and psychological (nine items) impact of MS on the patient’s previous 2-week day-to-day life.14,15 The Modified Fatigue Impact Scale 5-Item (MFIS-5) consisted of five items describing how fatigue affected a person.16 Treatment Satisfaction Questionnaire for Medication 14-item (TSQM-14) measures the patient’s level of satisfaction/dissatisfaction with medication (14 items);17 only patients with previous treatment were assessed for TSQM-14. EuroQol5D 5 level version (EQ-5D-5L) included two components: the EQ-5D, a descriptive system of the patient’s health state profile in five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), and the EQ-5D VAS, a quantitative measure in which patients described their health on that day on a scale from 0 to 100.18 For each patient, EQ-5D scores were derived based on value sets for England.18 Patient-reported Indices for Multiple Sclerosis activity limitations (PRIMUS-Activity Limitations) assessed the patient’s ability to perform various activities of daily living during the previous week without the use of aids (e.g. cane, walker, or wheelchair) or assistance (15 items).19,20 Work Productivity and Activity Impairment Multiple Sclerosis Version (WPAI-MS) assessed employment status and, during the previous 7 days, hours worked (if employed), hours of missed work due to MS or other reasons, effect on productivity due to MS while working, and activity impairment attributable to health problems (six items);21 WPAI-MS scores are only reported for patients who were employed at the time of PRO administration. Beck Depression Inventory-Fast Screen (BDI-Fast Screen) evaluated depression in patients with medical illness during the prior 2 weeks (seven items).22
Early MS subgroups
Three early MS subgroups were pre-specified in the statistical analysis plan as an exploratory endpoint: (a) Newly diagnosed: diagnosed with MS ≤1 year before study entry, naive to approved MS therapies; (b) EDSS:23 baseline EDSS score ≤3.5; and (c) Relapse: ≤1 relapse in the prior year.
Statistical analysis
An MS relapse was defined as new or recurrent neurological symptoms not associated with fever or infection, lasting ≥24 hours, accompanied by new objective neurological findings upon examination by the Investigator, followed by 30 days of stability or improvement (new or recurrent neurological symptoms that occurred < 30 days following the onset of a protocol-defined relapse were considered part of the same relapse). ARR was calculated as the total number of relapses in a period divided by the total patient-years of exposure in that period. The primary analyses for ARR, proportion of relapse, and PROs were conducted in the primary analysis population, comprising all eligible subjects, who provided signed informed consent, enrolled, and took ≥1 dose of DMF. Ninety-five percent confidence internals (CIs) of unadjusted ARR and rate ratio were estimated based on robust standard errors derived from an unadjusted Poisson regression model using the generalized estimating equation method. The proportion of patients relapsed from Baseline to 12 months was estimated using the Kaplan–Meier method based on time-to-first-relapse survival distribution. If patients did not experience a relapse prior to DMF discontinuation, they were censored on the last date known to be in the study. Mean and median change in PROs from Baseline to 12 months was assessed using a Wilcoxon signed-rank test; only patients with no missing data were included in the tabulation. The frequency of patients with non-missing data at each time point was presented. In general, if there was < 15% of missing data at any time point, data for that specific endpoint were imputed and the primary analyses were based on patients with non-missing data. If there was >15% missing data at any time point, analysis was based on a mixed-effects model.
The estimated proportion of patients with 24-week confirmed disability progression was based on the Kaplan–Meier product limit method, up to 52 weeks. Confirmed EDSS disability progression was defined as ≥1.0 point increase in EDSS from a baseline EDSS ≥1.0, or ≥1.5 point increase from a baseline EDSS of 0, or ≥0.5 point increase from a baseline EDSS ≥6 and confirmed at 6 months (154 days) after initial progression. Progression could start at a scheduled assessment or relapse assessment during the treatment period but could be confirmed during either the treatment and/or follow-up 24-week period. EDSS assessments during relapse assessments were not used for confirmation. Patients were censored at the earliest of the last EDSS assessment date during the treatment period or the last dose of DMF if they did not have progression.
Analysis methods for the subgroups are consistent with the primary analysis. For the association between change from baseline of the PROs and resource utilization (visit with neurologist: Yes/No) and treatment adherence (≥80% vs. < 80%), mixed-model repeated measures method was adjusted for baseline PRO values, baseline EDSS (≤3.5 vs. >3.5), visit and visit by the factor of interest interaction term in the model. All analyses were performed using SAS® v9.2 or higher (SAS Institute, Cary, NC, USA).
Standard protocol approvals, registrations, and patient consents
The study was approved by central and local ethics committees and conducted in accordance with International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent.
Results
Patients
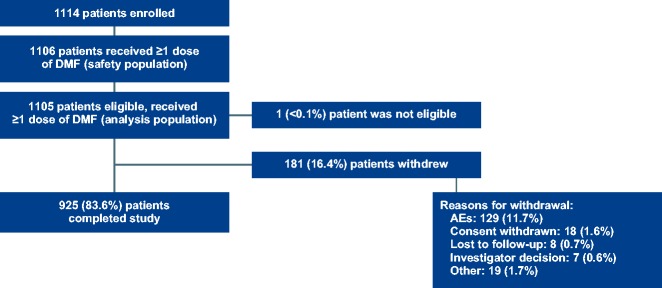
A total of 1105 (1114 enrolled) patients were included in the primary analysis population (Figure 1). Most baseline characteristics were similar to the phase 3 pivotal studies;6,7 however, there were notable differences for PROTEC (Table 1) versus the combined DEFINE/CONFIRM populations24 for relapses in the year before study enrollment (0.6 vs. 1.3) and percentage of patients with any prior MS medication (77% vs. 48%). Median (min, max) duration of DMF treatment was 12.0 (0.03, 16.3) months; the majority of patients (941/1106, 85%) remained on DMF treatment for >9 months. Mean (SD) overall adherence (weeks with full prescribed dose taken divided by the total 0 weeks during follow-up) was 80 (21%); 16% of patients did not take the full prescribed dose during the study due to side effects.
Figure 1.
Patient disposition for the PROTEC study is shown.
AE: adverse events, DMF: delayed-release DMF.
Table 1.
Patient baseline characteristics.
| Characteristic | TotalN = 1105 | Newly diagnosedn = 184 | EDSS ≤3.5n = 978 | Relapse ≤1n = 993 |
|---|---|---|---|---|
| Mean (SD) age, y | 38.8 ±10.0 | 35 (10) | 38 (10) | 39 (10) |
| Age category, y, n (%) | ||||
| 18–19 | 17 (2) | 6 (3) | 16 (2) | 14 (1) |
| 20–29 | 202 (18) | 63 (34) | 199 (20) | 164 (17) |
| 30–39 | 367 (33) | 60 (33) | 338 (35) | 329 (33) |
| 40–49 | 363 (33) | 39 (21) | 311 (32) | 338 (34) |
| 50–59 | 124 (11) | 13 (7) | 93 (10) | 118 (12) |
| ≥60 | 32 (3) | 3 (2) | 21 (2) | 30 (3) |
| Female, n (%) | 805 (72) | 116 (63) | 704 (72) | 718 (72) |
| Relapses in year before study enrolment, mean (SD) | 0.6 (0. 7) | 1.1 (0.7) | 0.6 (0.7) | 0.5 (0.5) |
| Time since most recent pre-study relapse, mean (SD), ya | 4.7 (3.3) | 4.1 (3.0) | 4.8 (3.3) | 5.1 (3.4) |
| Time since diagnosis of MS, mean (SD), y | 6.6 (6.1) | 0.4 (0.5) | 6.0 (5.5) | 6.9 (6.1) |
| EDSS score, median (range) | 2.0 (0, 7.0) | 1.6 (0, 6.5) | 1.6 (0, 3.5) | 2.0 (0.7) |
| Patients with any prior MS disease-modifying therapy n (%)b,c | 828 (75) | 2 (1) | 722 (74) | 763 (77) |
| Interferon beta-1a | 499 (45) | 0 (0) | 442 (46) | 460 (45) |
| Glatiramer acetate | 318 (28) | 0 (0) | 276 (29) | 293 (29) |
| Interferon beta-1b | 175 (16) | 0 (0) | 139 (15) | 157 (15) |
| Median (min, max) time since last MS disease-modifying therapy discontinuation, mo | 0.57 (0.03, 363.2) | |||
| Reason for prior MS treatment discontinuation, n (%)d | ||||
| Tolerability | 556 (50) | 0 (0) | 495 (52) | 519 (51) |
| Efficacy reasons | 335 (30) | 0 (0) | 266 (28) | 291 (29) |
| Safety | 58 (5) | 1 (50) | 50 (5) | 53 (5) |
| Other | 167 (15) | 1 (50) | 145 (15) | 156 (15) |
Total are patients in the primary analysis population.
EDSS: Expanded Disability Status Scale; values represent mean (SD) unless stated otherwise.
Race was not reported in 1001 patients due to confidentiality reasons implemented during the study.
aBased on data from 583 patients.
bOnly MS therapies taken before the first dose date of DMF initiation were included; patients may have received >1 therapy for MS.
cMedications previously taken by >10% of patients are shown. Other medications taken by >1% of patients include azathioprine, interferon beta, interferon, and teriflunomide.
dIf patients have >1 discontinuation reason for same MS therapy, only the last reason was used.
ARR
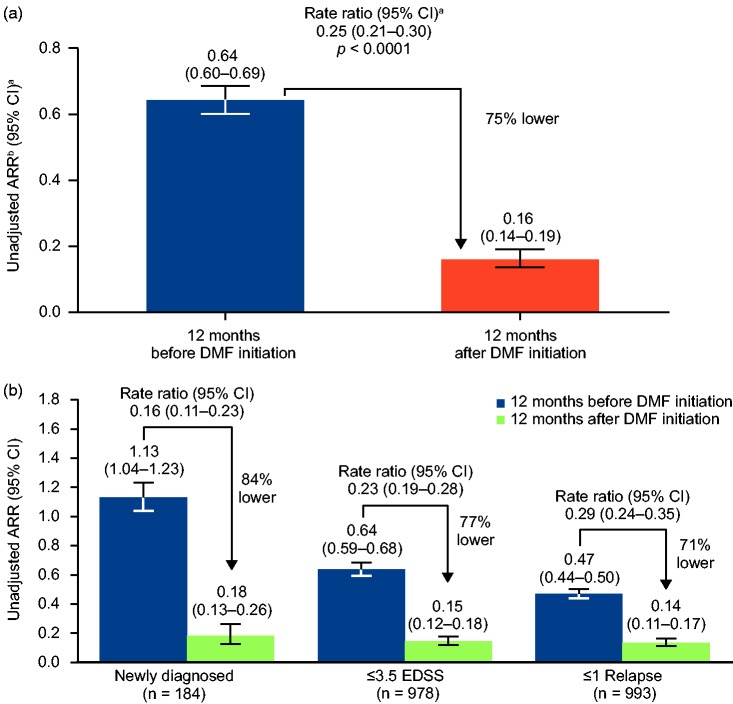
Unadjusted ARR at 12 months after DMF treatment initiation was 75% lower than for 12 months before DMF initiation (0.161 vs. 0.643, p < 0.0001; Figure 2); and 88% of patients were relapse-free 12 months after DMF initiation versus 48% at 12 months before DMF initiation. A total of 710 relapses were experienced in 579 patients in the 12 months prior to initiating DMF, versus 155 relapses in 135 patients in the 12 months after treatment with DMF. Based on Kaplan–Meier estimates, 88% of patients were relapse-free 12 months after DMF initiation. At 1 year following DMF initiation, 21 (1.9%) patients had 24-week confirmed disease progression prevalence; the cumulative incidence estimated proportion was 2.2% by Kaplan–Meier analysis. Among patients who experienced a relapse requiring IV steroid therapy, 97 (9%) experienced one, 11 (1%) experienced two, and one ( < 0.1%) experienced three relapses. Median (min, max) EDSS scores showed minor improvements from Baseline, 2.0 (0.0, 7.0), to Month 12, 1.5 (0.0, 7.0), p = 0.0136.
Figure 2.
ARR at 12 months before and after DMF initiation for (A) all patients and (B) early MS subgroups.
aBased on empirical (robust) SE from a generalized estimating equation using an unadjusted Poisson regression model.
bARR was calculated as the total number of relapses that occurred during that period of time for all patients, divided by the total number of patient-years followed in that period.
Subgroups are defined as: EDSS = baseline EDSS score ≤3.5; Relapse = ≤1 relapse in the prior year.
DMF: delayed-release DMF.
PROs
A total of 84% of patients treated with DMF completed the 12-month follow-up period. Overall, patients generally demonstrated improvement across several PRO measures compared with baseline (Table 2). For MSIS-29, measuring the physical and psychological impact of MS of the patient’s day-to-day life, there was significant improvement in mean (SD) scores between Baseline and Month 12 for physical: 22.8 (21.5) versus 18.3 (19.8), and psychological: 34.8 (23.7) versus 25.6 (22.2), p < 0.0001 for both comparisons. For MFIS-5, assessing the effects of fatigue on physical, cognitive, and psychosocial functioning, there was significant improvement in mean (SD) scores between Baseline and Month 12, 8.2 (5.0) versus 6.2 (5.1), p < 0.0001. Similarly, mean (SD) scores between Baseline and Month 12 scores in several WPAI measures improved, measuring impairment in work and activities due to MS: overall work impairment in the past 7 days, 23.7 (27.1) versus 19.1 (24.7), p = 0.0081; activity impairment in the past 7 days, 28.5 (27.8) versus 22.8 (26.0), p < 0.0001.
Table 2.
Mean change in PROs from baseline to 12 months.
| Measure | Description | Component |
Mean (SD) change from baseline to Month 12 |
|||
|---|---|---|---|---|---|---|
| Improved | Stable | Worsened | p-value | |||
| MSIS-29a | 20 items assess physical impact of MS in terms of mobility and self-care | Physical impactn = 868 | +–3.0 (14.1) | <0.0001 | ||
| 9 items assess psychological impact of MS | Psychological impactn = 860 | +–8.0 (18.6) | <0.0001 | |||
| MFIS-5b | 5 items assess how fatigue impacts patients’ lives | n = 867 | +–1.7 (3.8) | <0.0001 | ||
| TSQM-14c | 14 items assess patient treatment satisfaction | Effectivenessn = 492 | +14.4 (29.5) | <0.0001 | ||
| Side effectsn = 500 | +25.4 (31.4) | <0.0001 | ||||
| Conveniencen = 505 | +33.4 (25.7) | <0.0001 | ||||
| Global scoren = 511 | +21.8 (26.9) | <0.0001 | ||||
| EQ-5D-5Ld | 5 items assess mobility, self-care, usual activities, pain and discomfort, anxiety and depression | EQ-5Dn = 792 | +0.02 (0.1) | <0.0001 | ||
| EQ-5D VASn = 803 | +3.7 (15.9) | <0.0001 | ||||
| PRIMUS activity limitationse | 15 items assess activities of daily living | n = 545 | ±–0.14 (3.2) | 0.2134 | ||
| WPAI-MSf | 6 items assess the number of work hours missed, impact on productivity, and daily activities during past 7 days | Work impairmentfn = 327 | +–2.2 (19.5) | 0.008 | ||
| Activity impairmentn = 851 | +–4.2 (22.3) | <0.0001 | ||||
| BDI-Fast Screeng | 7 items assess depression during the past 2 weeks | n = 854 | +–0.8 (2.5) | <0.0001 | ||
| EDSSh | Assesses disease progression | n = 940 | ±–0.05 (0.65) | 0.0136 | ||
BDI-Fast Screen: Beck Depression Inventory-Fast Screen; EDSS: Expanded Disability Status Scale; EQ-5D-5L: EuroQol-5D 5-level version; MFIS-5: Modified Fatigue Impact Scale 5-item version; MSIS-29, Multiple Sclerosis Impact Scale; PRIMUS: Patient-Reported Indices for Multiple Sclerosis; PRO: patient-reported outcome; TSQM-14: Treatment Satisfaction Questionnaire for Medication; WPAI-MS: Work Productivity and Activity Impairment for Multiple Sclerosis.
Mean change in PROs from baseline to 12 months was assessed using a Wilcoxon signed-rank test.
aLower score indicates better outcome; range, 0–100 (scores were transformed).
bLower score indicates improved functioning; range, 0–20.
cHigher score indicates greater satisfaction; range, 0–100; only patients with previous treatment were assessed.
dHigher score indicates better health; range, 0–100.
eHigher score indicates better outcome; range, 0–30.
fHigher score indicates higher impairment and lower productivity; scores are only reported for patients who were employed at the time of PRO administration.
gLower score indicates less severe depressive symptoms; range, 0–21.
hLower score indicates less severe disability symptoms; range, 0–10.
Other PRO measures showed small but significant functional differences in scores. TSQM-14 scores (patient satisfaction with their current medication) improved significantly between Baseline and Month 12 for the global score and its components: TSQM-14 global score, 51.4 (22.5) versus 73.6 (21.0); effectiveness, 54.3 (21.7) versus 70.1 (24.7); side effects, 60.1 (30.6) versus 85.4 (19.4); convenience, 52.4 (21.2) versus 85.5 (17.2), p < 0.0001 for all comparisons. There were significant improvements from Baseline to Month 12 for EQ-5D, 0.8 (0.2) versus 0.9 (0.2); EQ-5D VAS, 74.1 (18.7) versus 79 (17.5); and BDI-FAST, assessing depressive symptoms, 2.8 (3.2) versus 1.9 (3.0), p < 0.0001 for all comparisons.
PRIMUS-Activity Limitations scores remained stable between Baseline and Month 12, mean (SD) 3.1 (4.6) at Baseline versus 2.6 (4.3) at Month 12, suggesting no significant change in patients’ ability to perform various activities of daily living without aids or assistance. There was no evidence of change in PRO scores between Baseline and Month 12 for patients who visited a neurologist and those who did not. Similarly, there was no evidence of a change in PRO scores between Baseline and Month 12 patients with < 80% adherence versus ≥80% adherence.
Early MS and other subgroups: Relapses and PRO
The effectiveness of DMF in newly diagnosed patients and different levels of disability was analyzed by dividing the population into subgroups. Among the patients who received ≥1 dose of DMF, 184 were newly diagnosed, 978 in the EDSS ≤3.5 subgroup, and 993 in the ≤1 relapse subgroup. Similar to the overall population, ARR at 12 months after DMF initiation was 84% lower than ARR estimated for the 12 months before DMF initiation in newly diagnosed patients, 77% in the EDSS ≤3.5 subgroup, and 71% in the ≤1 relapse subgroups, p < 0.0001 for all subgroup (Figure 2(b)). Based on Kaplan–Meier estimates, the percentages of patients relapse-free 12 months after DMF initiation were 84.2% for newly diagnosed patients, 87.7% in the EDSS ≤3.5 subgroup, and 88.4% in the ≤1 relapse subgroup.
Significant improvements in scores from baseline to 12 months were observed across all early MS subgroups for MSIS-29, MFIS-5, EQ-5D, and EQ-5D VAS (Table 3).
Table 3.
Change in PROs from baseline to 12 months in early MS subgroups.
| Measure | Description | Component | Newly diagnosed(n = 184) | EDSS ≤3.5(n = 978) | Relapse ≤1(n = 993) |
|---|---|---|---|---|---|
| MSIS-29a | 20 items assess physical impact of MS in terms of mobility and self-care | Physical impact | +–3.1 (14.8) | +–2.7 (13.4) | +–3.2 (14.2) |
| 9 items assess psychological impact of MS | Psychological impact | +–9.1 (16.9) | +–8.0 (18.2) | +–8.1 (18.5) | |
| MFIS-5b | 5 items assess how fatigue impacts patients’ lives | +–1.6 (3.5) | +–1.7 (3.7) | +–1.7 (3.8) | |
| EQ-5D VASc | 5 items assess mobility, self-care, usual activities, pain and discomfort, anxiety and depression | +4.1 (13.7) | +3.6 (15.1) | +3.7 (15.9) |
EDSS: Expanded Disability Status Scale; EQ-5D VAS: EQ-5D visual analogue scale; MFIS-5: Modified Fatigue Impact Scale 5-item version; MSIS-29: Multiple Sclerosis Impact Scale; PRO: patient-reported outcome.
+ = Statistically significant improvement; ± = no statistically significant change; – = statistically significant worsening.
aLower score indicates better outcome; range, 0–100 (scores were transformed).
bLower score indicates improved functioning; range, 0–20.
cHigher score indicates better health; range, 0–100.
Safety
Overall, 914 (83%) patients experienced an AE; serious AEs occurred in 41 (4%) patients (Table 4). One death occurred due to an accident, deemed unrelated to DMF treatment. A total of 126 (11%) patients discontinued DMF treatment due to AEs. The most common treatment-related AEs leading to DMF discontinuation (≥1% of patients) were abdominal pain (2%), diarrhea (1%), and vomiting (2%).
Table 4.
Summary of adverse events.
| AE, n (%) | Totaln = 1106 |
|---|---|
| Any AE | 914 (83) |
| Most common AEs in ≥10% patients | |
| Flushing | 477 (43) |
| Diarrhea | 172 (16) |
| Abdominal pain upper | 120 (11) |
| Abdominal pain | 115 (10) |
| Any SAE | 41 (4) |
| Most frequently occurring SAEs in ≥2 patients | |
| Fall | 4 (<1) |
| Lymphopenia | 3 (<1) |
| Breast cancer | 2 (<1) |
| MS relapse | 2 (<1) |
AE: adverse event; SAE: serious adverse event.
No cases of progressive multifocal leukoencephalopathy were reported. Mean absolute lymphocyte counts at 12 months (1.26 × 109/l) remained above the lower limit of normal (0.91 × 109/l) (Table 5), consistent with trial data.6,7 Of the 400 patients with ≥2 post-baseline lymphocyte assessments, 44 (11%) had moderate, persistent lymphopenia (≥2 consecutive lymphocyte levels of < 0.8 × 109/l 180 days apart), and four (1%) had severe, persistent lymphopenia (two consecutive lymphocyte levels of < 0.5 × 109/l 180 days apart).
Table 5.
Lower lymphocyte counts.
| Total | |
|---|---|
| Mean (SD) lymphocyte counts, × 109/l | 1.26 (0.55) |
| Baseline, n = 1038 | 1.93 (0.61) |
| Month 12, n = 897 | 1.26 (0.55) |
| 0.8 – <0.91 (LLN), n/N (%)a | 69/831 (8) |
| 0.5 – <0.8, n/N (%)a | 133/831 (16) |
| <0.5, n/N (%)a | 17/831 (2) |
| Patients with ≥2 post-baseline lymphocyte assessments, n | 400 |
| Moderate, persistent lymphopeniab, n | 44 |
| Severe, persistent lymphopeniac, n | 4 |
ALC: absolute lymphocyte count; LLN: lower limit of normal.
aFor patients with at least 1 baseline assessment >LLN.
bALC of at least 2 consecutive lymphocyte levels of <0.8 × 109/L 180 days apart.
cALC of at least 2 consecutive lymphocyte levels of <0.5 × 109/L 180 days apart.
Discussion
The results reported are consistent with DMF effectiveness data from other real-world phase 4 studies (RESPOND; NCT01903291),25 supporting the findings from phase 3 studies of DMF.6,7 Unadjusted ARR at 12 months after DMF treatment initiation was significantly lower compared with 12 months before DMF initiation. The majority of patients were relapse-free 12 months after DMF initiation. Safety and tolerability were consistent with the known safety profile of DMF.3,4,6,7,24 Lymphocyte counts were similar to those reported in pivotal clinical trials and clinical practice.6,7,26–28 Statistically significant improvements from baseline to 12 months were observed for the majority of PROs, indicating that DMF is an effective MS treatment from a patient perspective as well as based on classical clinical outcomes.
Clinical significance of PROs scores
In patients with MS and other chronic diseases, a widely used threshold for discrimination of meaningful change in HRQoL is 1/2 SD change in PRO score over 1 year.29 In a population of patients with RRMS, a ≥7.50 point worsening was determined to be a practical threshold for identifying a clinically significant change in the physical impact of MS using the MSIS-29 physical score.30 The threshold for worsening was defined as < –0.074 points for EQ-5D31 and < –5.5 points for EQ-5D VAS,32 although these thresholds were not calculated in patients with MS. In this analysis, the mean change was –3.0 for MSIS-29 physical score, 0.02 for EQ-5D, and 3.7 for EQ-5D VAS: statistically significant, although they may not be meaningful clinical changes based on criteria described in previous studies. By the 1/2 SD rule, MFIS-5 (mean –1.7; SD 3.8), TSQM-14 side effects, TSQM-14 convenience, and TSQM-14 global score reached clinically significant change.
Early MS treatment has been associated with a reduction in disease progression.33,34 When considering the optimal treatment for reducing disease progression risk in patients with early MS, relapses, MRI lesions, disability progression, and brain volume loss are four key measures of disease activity.35 For treatment-naïve patients, IFN-β, glatiramer acetate, teriflunomide, and DMF, followed by fingolimod, natalizumab, and alemtuzumab, have been suggested in patients with breakthrough disease activity.35 ARR and PRO results for the newly diagnosed subgroup and the subgroups with EDSS ≤3.5 or relapse ≤1 in the prior year were similar to the overall group, indicating DMF is effective in a broad range of patients.
The results and interpretations are subject to several limitations common to real-world studies, including the observational nature of the study, short duration of follow-up time on DMF, and lack of active comparators. Potential bias arises due to regression to the mean because the majority of patients enrolled had previous experience with disease-modifying therapies (DMTs) for MS. Therefore, familiarity to compliance with treatment and dosage requirements impact the generalizability of results for treatment-naïve or less adherent patients. Due to the real-world nature of the study, eligibility criteria did not restrict participation to patients with ≥1 relapse over the 12-month period prior to study entry, as in DEFINE and CONFIRM, rendering it possible that patients in this study had a less severe progression of MS at enrollment. Therefore, these patients may have exhibited lower risk possibly resulting in lower incidence of relapse over the study period, regardless of treatment. The study design may impede researchers dictating when PRO assessments are completed relative to relapses. PRO assessments obtained shortly after relapse have a greater risk of being affected by the relapse compared with PROs obtained with no recent relapse, although comparisons of PRO outcomes in patients with or without relapses during the study found either a marginal difference or no difference in the change from Baseline to Month 12. Missing data from patient-reported measures is a limitation of real-world studies; however, mitigating the risk of missing data impacting study results, the proportion of missing data was described and accounted for throughout the statistical analyses. Unfortunately, the influence of patients “not missing at random” may introduce bias in both the estimate and variance for which the sensitivity analysis cannot account. To minimize potential bias due to methods of data collection, case report forms were carefully designed, with clear instructions and training provided to site staff; furthermore, mitigations were carried out if data entry concerns were identified. Finally, the 11.7% of patients who discontinued due to AEs may introduce possible confounding or bias in the QoL measures, given the symptomatic nature of some AEs and their impact of QoL outcomes.
Comparing treatment-satisfaction scores between previously discontinued drugs with treatment-satisfaction scores in drugs not discontinued for ≥12 months could be biased in favor of DMF purely because of patient selection. Sensitivity analyses were performed for primary and secondary endpoints using patient populations who completed the full study period (completer population), in order to evaluate potential effects of early study withdrawal on analytic results. Results were similar between the completer and analysis populations. Also, since the exclusions criteria were only based on last DMT, the assessment may be limited if patients were on multiple DMTs and omitting the type or strength of DMT.
Conclusions
Overall, the results suggest that DMF may offer an effective treatment option for patients with RRMS, both from a clinical perspective and based on patients’ satisfaction with DMF treatment in relation to their personal quality of life, health status, and physical abilities.
Acknowledgements
Biogen provided funding for medical writing support in the development of this paper; Ana Antaloae, PhD and Karen Spach, PhD from Excel Scientific Solutions wrote the first draft of the manuscript based on input from authors, and Elizabeth Wassmer from Excel Scientific Solutions copyedited and styled the manuscript per journal requirements. Biogen reviewed and provided feedback on the paper. The authors had full editorial control of the paper, and provided their final approval of all content.
Contributor Information
T Berger, Universitätsklinik für Neurologie, Medizinische Universität, Austria.
B Brochet, Groupe Hospitalier Pellegrin Hôpital Pellegrin, France.
L Brambilla, IRCCS Foundation Neurological Institute Carlo Besta, Italy.
PS Giacomini, Montreal Neurological Institute & Hospital, McGill University Health Center, Canada.
A Vasco Salgado, Hospital Professor Doutor Fernando Fonseca, E.P.E., Portugal.
R Su, Biogen, USA.
A Bretagne, Biogen International GmbH, Switzerland.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. Requests for data supporting this manuscript should be submitted to the Biogen Clinical Data Request Portal (www.biogenclinicaldatarequest.com).
Conflict of interests
TB: consulting/speaker fees from Almirall, AOP Pharma, Biogen, Bionorica, Celgene, MedDay, Merck, Novartis, Roche, Sanofi/Genzyme, and Teva; his institution (Universitätsklinik für Neurologie) has received honoraria for study participation and unrestricted research grants from Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi/Genzyme, and Teva; BB: advisory boards for Biogen, Genzyme, Merck-Serono, Novartis, and Roche; his institution (Groupe Hospitalier Pellegrin Hôpital Pellegrin) has received support for clinical trials and research activities from Actelion, Bayer HealthCare, Biogen, Genzyme, Merck Serono, MedDay, Novartis, Roche, and Teva; LB: received honoraria for speaking from Novartis and for traveling from Sanofi-Genzyme and Roche; she acted as an Advisory Board member of Sanofi-Genzyme and principal investigator in clinical trials for Roche; sub-investigator in clinical trial for Novartis, Biogen, Sanofi-Genzyme, Merck; XM: received speaking honoraria and travel expenses for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Actelion, Bayer, Biogen, Celgene, Excemed, Genzyme, Merck, MSIF, NMSS, Novartis, Roche, Sanofi-Genzyme, and Teva Pharmaceutical; PSG: consulting/speaker fees from/advisory boards for Actelion, Allergan, Bayer, Biogen, EMD Serono, Merz, Novartis, Pendopharm, Roche, Sanofi-Genzyme, and Teva Neuroscience; research support from Biogen; consultant for NeuroRx Research; principal investigator/sub-investigator for clinical trials for Actelion, Alexion, Bayer HealthCare, Biogen, Elan, EMD Serono, GlaxoSmithKline, MedImmune, Novartis, Ono, Roche-Genentech, Sanofi-Aventis, and Teva Neuroscience; AVS: consultant for Biogen, Merck Serono, Novartis, and Sanofi-Genentech; fees from Roche and Boehringer Ingelheim; RS and AB: employees of and hold stock/stock options in Biogen
Funding
This study was supported by Biogen.
ORCID iDs
T Berger https://orcid.org/0000-0001-5626-1144
PS Giacomini https://orcid.org/0000-0002-1346-3042
References
- 1.Nortvedt MW, Riise T. The use of quality of life measures in multiple sclerosis research. Mult Scler 2003; 9(1): 63–72. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande PR, Rajan S, Sudeepthi BL, Abdul Nazir CP. Patient-reported outcomes: A new era in clinical research. Perspect Clin Res 2011; 2(4): 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.TECFIDERA. (dimethyl fumarate) [prescribing information]. Cambridge, MA 02142 Biogen Inc; Rev 4/2015.
- 4.Biogen. PRODUCT MONOGRAPH TECFIDERA. 2015.
- 5.Biogen. Dimethyl fumarate Package leaflet: Information for the patient. 2015.
- 6.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367(12): 1087–1097. [DOI] [PubMed] [Google Scholar]
- 7.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367(12): 1098–1107. [DOI] [PubMed] [Google Scholar]
- 8.Gold R, Giovannoni G, Phillips JT, et al. Sustained effect of delayed-release dimethyl fumarate in newly diagnosed patients with relapsing-remitting multiple sclerosis: 6-year interim results from an extension of the DEFINE and CONFIRM studies. Neurol Ther 2016; 5(1): 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kita M, Fox RJ, Gold R, et al. Effects of delayed-release dimethyl fumarate (DMF) on health-related quality of life in patients with relapsing-remitting multiple sclerosis: An integrated analysis of the phase 3 DEFINE and CONFIRM studies. Clin Ther 2014; 36(12): 1958–1971. [DOI] [PubMed] [Google Scholar]
- 10.Lee A, Pike J, Edwards MR, et al. Quantifying the benefits of dimethyl fumarate over beta interferon and glatiramer acetate therapies on work productivity outcomes in MS patients. Neurol Ther 2017; 6(1): 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodin DS, Bates D. Treatment of early multiple sclerosis: The value of treatment initiation after a first clinical episode. Mult Scler 2009; 15(10): 1175–1182. [DOI] [PubMed] [Google Scholar]
- 12.Gold R, Giovannoni G, Phillips JT, et al. Efficacy and safety of delayed-release dimethyl fumarate in patients newly diagnosed with relapsing-remitting multiple sclerosis (RRMS). Mult Scler 2015; 21(1): 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983; 33(11): 1444–1452. [DOI] [PubMed] [Google Scholar]
- 14.Hobart J, Lamping D, Fitzpatrick R, et al. The Multiple Sclerosis Impact Scale (MSIS-29): A new patient-based outcome measure. Brain 2001; 124(Pt 5): 962–973. [DOI] [PubMed] [Google Scholar]
- 15.Gray O, McDonnell G, Hawkins S. Tried and tested: The psychometric properties of the multiple sclerosis impact scale (MSIS-29) in a population-based study. Mult Scler 2009; 15(1): 75–80. [DOI] [PubMed] [Google Scholar]
- 16.Putzki N, Yaldizli O, Tettenborn B, et al. Multiple sclerosis associated fatigue during natalizumab treatment. J Neurol Sci 2009; 285(1-2): 109–113. [DOI] [PubMed] [Google Scholar]
- 17.Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2004; 2: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.EuroQol G. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy 1990; 16(3): 199–208. [DOI] [PubMed] [Google Scholar]
- 19.McKenna SP, Doward LC, Twiss J, et al. International development of the patient-reported outcome indices for multiple sclerosis (PRIMUS). Value Health 2010; 13(8): 946–951. [DOI] [PubMed] [Google Scholar]
- 20.Doward LC, McKenna SP, Meads DM, et al. The development of patient-reported outcome indices for multiple sclerosis (PRIMUS). Mult Scler 2009; 15(9): 1092–1102. [DOI] [PubMed] [Google Scholar]
- 21.Glanz BI, Degano IR, Rintell DJ, et al. Work productivity in relapsing multiple sclerosis: Associations with disability, depression, fatigue, anxiety, cognition, and health-related quality of life. Value Health 2012; 15(8): 1029–1035. [DOI] [PubMed] [Google Scholar]
- 22.Benedict RH, Fishman I, McClellan MM, et al. Validity of the Beck Depression Inventory-Fast Screen in multiple sclerosis. Mult Scler 2003; 9(4): 393–396. [DOI] [PubMed] [Google Scholar]
- 23.Ratzker P, Feldman J, Scheinberg L, et al. Self-assessment of neurologic impairment in multiple sclerosis. J Neuro Rehab 1997; 11: 207–211. [Google Scholar]
- 24.Viglietta V, Miller D, Bar-Or A, et al. Efficacy of delayed-release dimethyl fumarate in relapsing-remitting multiple sclerosis: Integrated analysis of the phase 3 trials. Ann Clin Transl Neurol 2015; 2(2): 103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kresa-Reahl K, Repovic P, Robertson D, et al. Effectiveness of delayed-release dimethyl fumarate on clinical and patient-reported outcomes in patients with relapsing multiple sclerosis switching from glatiramer acetate: RESPOND, a prospective observational study. Clin Ther 2018; 40(12): 2077–87. [DOI] [PubMed]
- 26.Fox RJ, Chan A, Gold R, et al. Characterizing absolute lymphocyte count profiles in dimethyl fumarate-treated patients with MS: Patient management considerations. Neurol Clin Pract 2016; 6(3): 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longbrake EE, Naismith RT, Parks BJ, et al. Dimethyl fumarate-associated lymphopenia: Risk factors and clinical significance. Mult Scler J Exp Transl Clin 2015; 1: 2055217315596994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longbrake EE, Cross AH. Dimethyl fumarate associated lymphopenia in clinical practice. Mult Scler 2015; 21(6): 796–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care 2003; 41(5): 582–592. [DOI] [PubMed] [Google Scholar]
- 30.Phillips GA, Wyrwich KW, Guo S, et al. Responder definition of the Multiple Sclerosis Impact Scale physical impact subscale for patients with physical worsening. Mult Scler 2014; 20(13): 1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 2005; 14(6): 1523–1532. [DOI] [PubMed] [Google Scholar]
- 32.Mathias S, Pritchard ML, Colwell HH, et al. What is the minimal clinically important difference and responsiveness of a patient-reported outcome questionnaire for metastatic colorectal cancer? Ann Oncol 2006; 17(Supplement 9): ix 121. [Google Scholar]
- 33.Comi G, Filippi M, Barkhof F, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: A randomised study. Lancet 2001; 357(9268): 1576–1582. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med 2000; 343(13): 898–904. [DOI] [PubMed] [Google Scholar]
- 35.Ziemssen T, De Stefano N, Sormani MP, et al. Optimizing therapy early in multiple sclerosis: An evidence-based view. Mult Scler Relat Disord 2015; 4(5): 460–469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. Requests for data supporting this manuscript should be submitted to the Biogen Clinical Data Request Portal (www.biogenclinicaldatarequest.com).