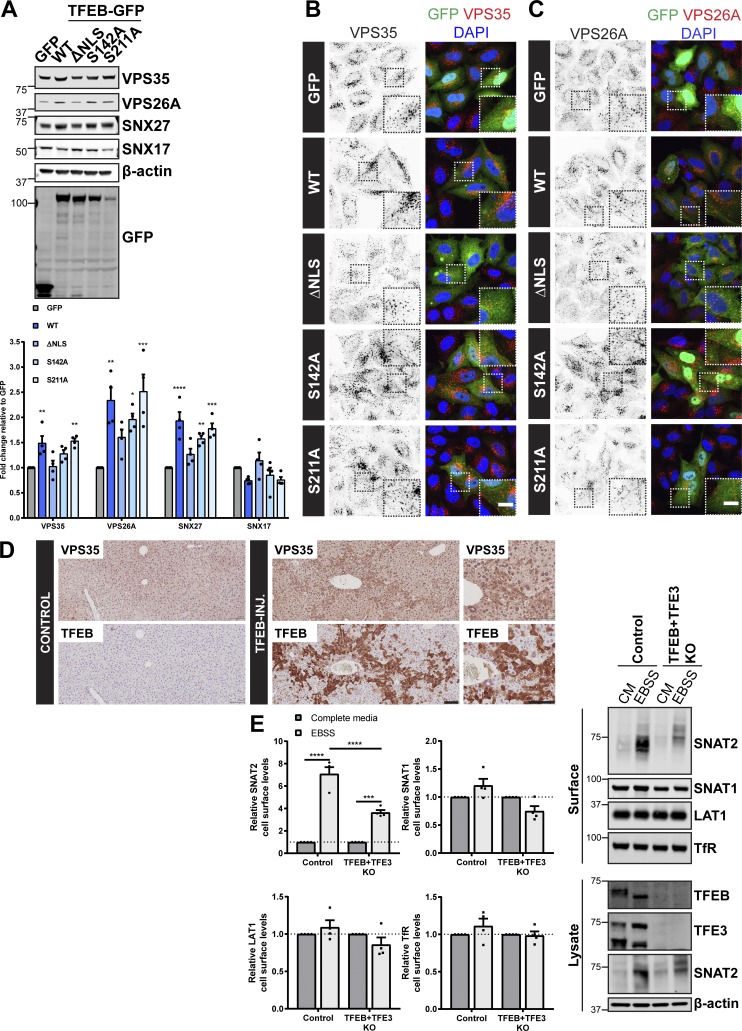
Figure 5.
TFEB regulates expression of the retromer complex in cellulo and in vivo. (A) HeLa cells were infected with lentiviruses expressing the indicated GFP-tagged TFEB or GFP alone. Cells were lysed, and total cell lysates were examined for the indicated proteins by immunoblotting. Data were normalized to β-actin. Quantification represents mean ± SEM fold change relative to GFP-expressing cells; n = four independent experiments; one-way ANOVA followed by Dunnett’s multiple comparison. (B and C) IF analysis of endogenous retromer component VPS35 (B) or VPS26A (C) in HeLa cells transiently transfected with the indicated GFP-tagged TFEB construct or GFP alone. (D) Immunohistochemistry of liver sections from mice starved for 24 h and injected with an adenoviral control vector (control) or human TFEB under control of a liver-specific promoter (TFEB-INJ). Tissues were stained for VPS35 or TFEB. Scale bars, 10 µm (B and C) and 100 µm (D). (E) Control and TFEB + TFE3 knockout HeLa cells were subjected to overnight (16 h) amino acid starvation (EBSS) or maintained in complete media (CM). Cells were surface biotinylated, and streptavidin agarose was used to capture biotinylated membrane proteins. Surface abundance of the indicated proteins was assessed by quantitative immunoblotting. The quantification shows the mean ± SEM; n = four independent experiments; two-way ANOVA followed by Sidak’s multiple comparison. (A and E) *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. KO, knockout.