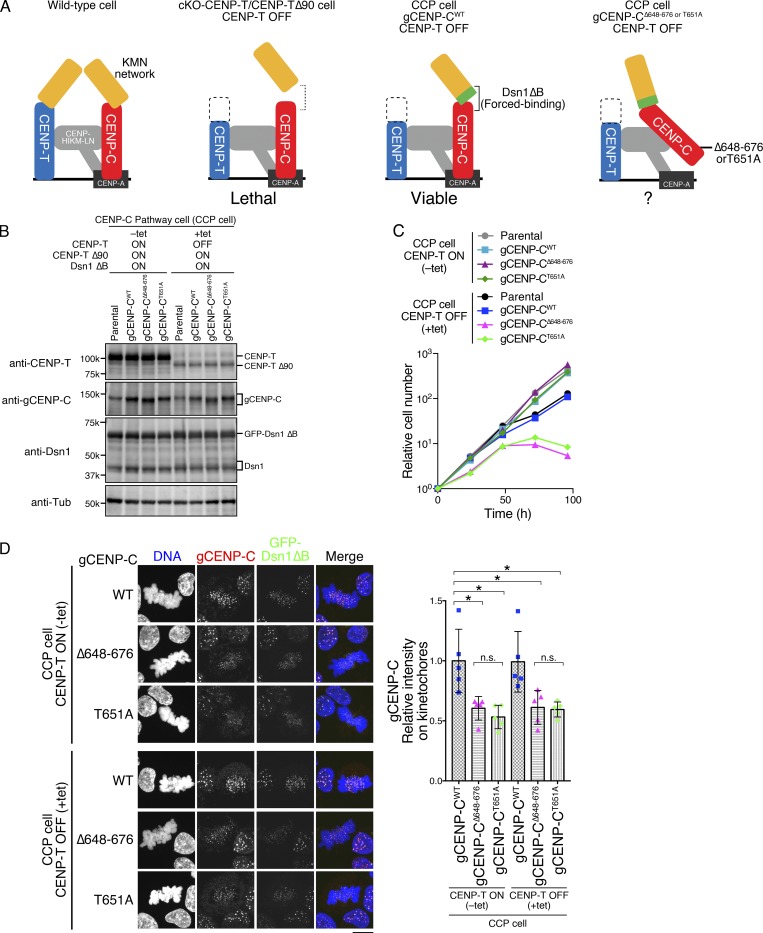
Figure 6.
CENP-A–CENP-C binding is essential for the CCP. (A) Schematic representation of kinetochores in WT and several mutant DT40 cell lines. In WT, the KMN network is recruited to kinetochores through two pathways: the CENP-C and CENP-T pathways (left). cKO-CENP-T cells expressing CENP-T Δ90 (cKO-CENP-T/CENP-T Δ90) died after inhibition of WT CENP-T expression by tet treatment (CENP-T OFF; second from left; Hara et al., 2018). This lethality is suppressed by expressing a Dsn1 mutant lacking the basic domain (aa 93–114; Dsn1 ΔB), which increases binding affinity of CENP-C to the KMN network (third from left: CCP cell; Hara et al., 2018). The CCP cells rely solely on the CCP to recruit the KMN network and enable us to test the importance of the CENP-A–binding domains in the CCP (right). (B) Expression of each gCENP-C construct (Fig. S2, D and E) in CCP cells cultured in the presence or absence of tet (+tet: CENP-T OFF or −tet: CENP-T ON) for 48 h. Expression of CENP-T, gCENP-C, and Dsn1 proteins was examined by immunoblotting. Parental CCP cells were also examined. α-Tubulin (Tub) was probed as a loading control. (C) Growth of CCP cells expressing gCENP-C WT, Δ648–676, or T651A. Cell proliferation was examined in the presence or absence of tet (+tet: CENP-T OFF or −tet: CENP-T ON) at the indicated time after tet addition. Parental CCP cells were also examined. Each graph shows the results of one experiment. (D) Localization of gCENP-C WT, Δ648–676, or T651A in CCP cells by immunostaining with an anti-gCENP-C antibody (red). GFP-Dsn1 ΔB shows kinetochores (green). DNA was stained by DAPI (blue). Scale bar indicates 10 µm. The CENP-C signal intensities on kinetochores in mitotic cells were quantified. Bar graph indicates mean with SD (n = 5; *, P < 0.05; **, P < 0.01; NS, P > 0.05, unpaired t test, two tailed). See also Fig. S3 F.