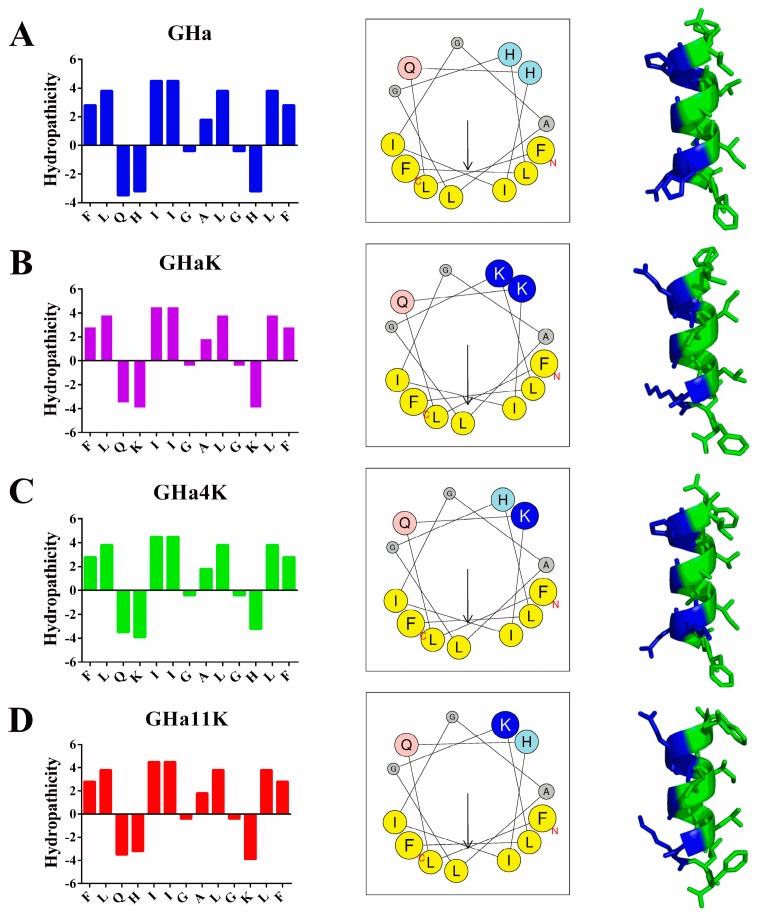
Figure 1.
The distribution of hydrophilic and hydrophobic amino acid residues, helical wheel projection and the predicted 3D structures. The four rows of (A), (B), (C), and (D) are GHa, GHaK, GHa4K, and GHa11K. The left column showed the amphipathic profile calculated by the ExPASy. The middle column showed the helical wheel projection of the peptides generated by using the Heliquest (http://heliquest.ipmc.cnrs.fr/). The arrows indicated the direction of the hydrophobic moment. The right column demonstrates the 3D structure, analyzed by using PEP-FOLD (http://bioserv.rpbs.univ-paris-diderot.fr/services/PEP-FOLD/), with a hydrophilic surface in blue and a hydrophobic surface in green.