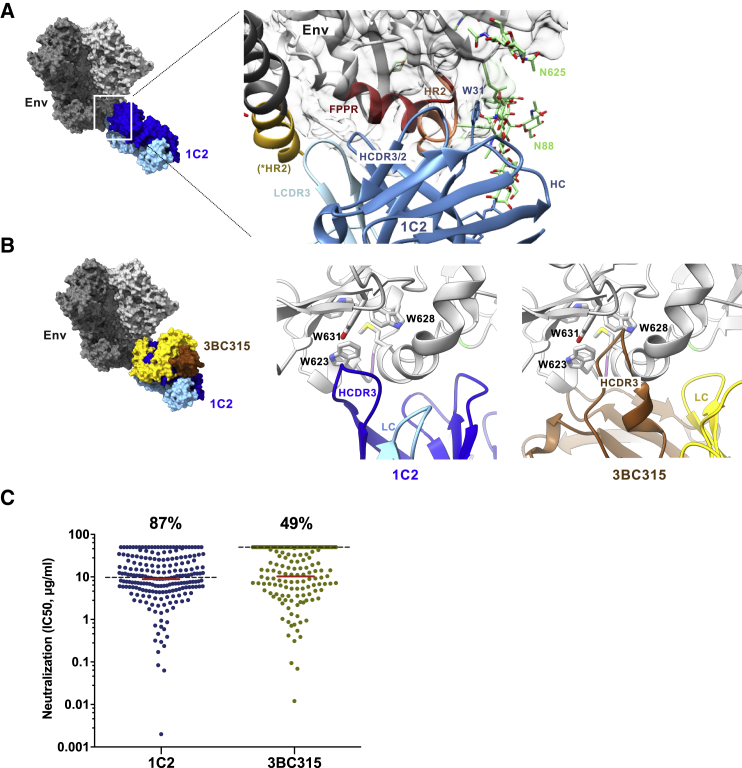
Figure 6.
Vaccine-Elicited bNAb 1C2 Is Similar to the Human bNAb 3BC315
(A) Reconstruction of 1C2 (blue) in complex with 16055 NFL TD 2CC+ Env trimer from the ∼3.9 Å resolution cryo-EM structure. Magnified view of the epitope is shown to the right. The 1C2 HC framework regions interact with glycan N88 and stabilize in a position close to glycan N625. The HCDRs and LCDR3 are involved in peptide contacts with gp41, primarily around the fusion peptide proximal region (FPPR) and HR2 helix. HCDR3 of 1C2 is near the tryptophan clasp of gp41, marked by residue W31. ∗HR2, from adjacent protomer.
(B) The medium resolution cryo-EM reconstruction of 3BC315 Fab in complex with BG505 SOSIP trimer (EMD: 3067) was aligned to the map of the 1C2-bound trimer complex. The docked crystal structure of 3BC315 Fab (PDB: 5CCK) is shown relative to 1C2. Magnified views of 1C2 interaction with the tryptophan clasp of gp41 are shown to the right compared to 3BC315.
(C) Neutralization potency (IC50, μg/mL) of 1C2 compared to 3BC315 in a 208-virus panel. Percentage of neutralized viruses are indicated at the top. Median IC50 of all viruses (gray dashed line) compared to sensitive viruses only (red line; non-neutralized viruses excluded) are shown. See also Figures S6 and S7 and Tables S5 and S6.