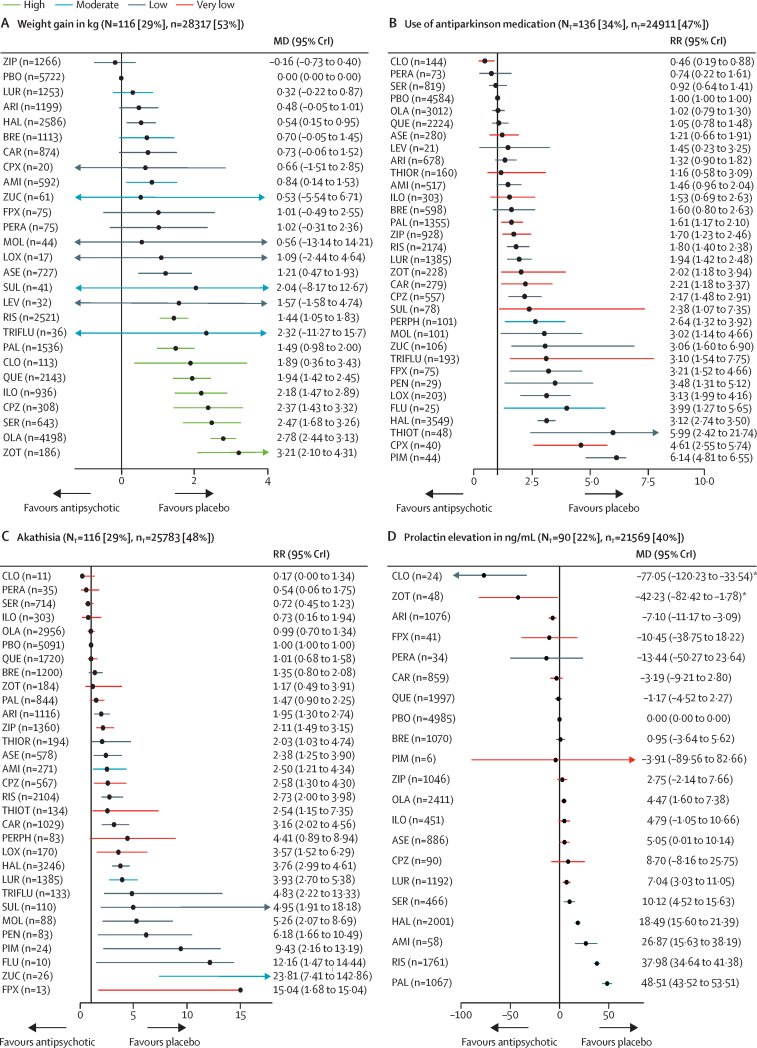
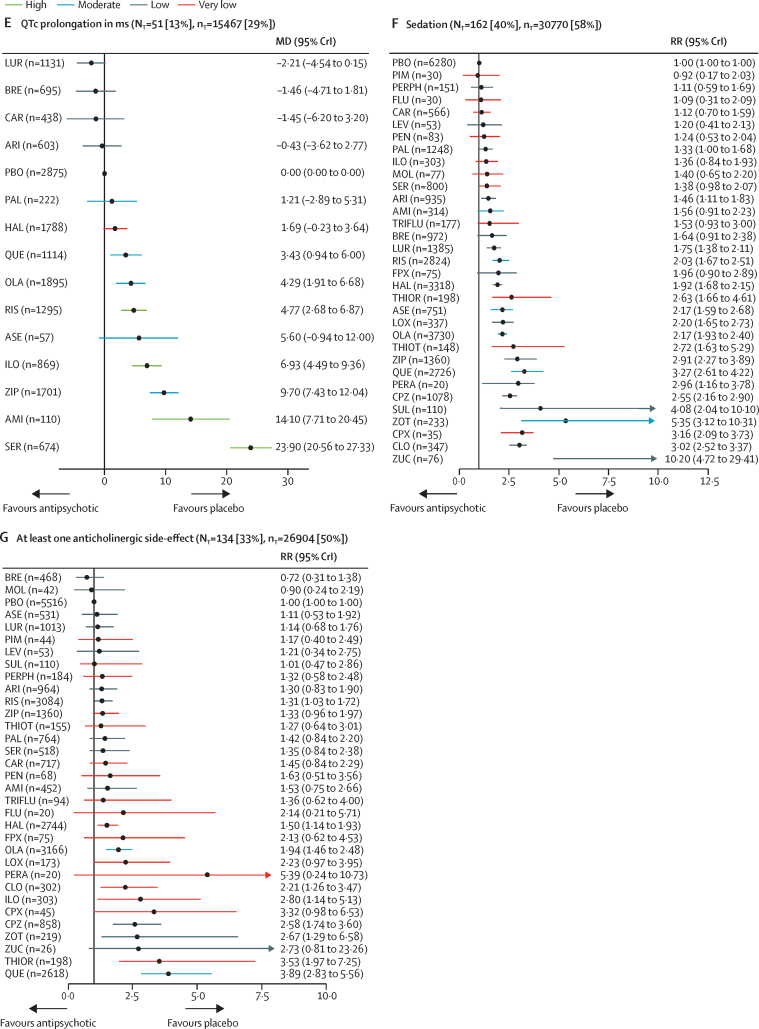
Figure 4.
Change in side-effect outcomes
(A) Weight gain in kg. (B) Use of antiparkinson medication. (C) Akathisia. (D) Prolactin elevation in ng/mL. (E) QTc prolongation in ms. (F) Sedation. (G) At least one anticholinergic side-effect. Treatments are ranked according to their surface under the curve cumulative ranking and compared with placebo. Effect sizes are presented as mean difference or risk ratio with 95% CrIs. The evidence is graded using CINeMA system (Confidence in Network Meta-Analysis), an adaption of the GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) approach for network meta-analysis. Colours indicate the confidence in the evidence: green=high, blue=moderate, grey=low, red=very low. NT=total number of trials reporting the outcome (percentage of sample). nT=total number of participants available for the respective outcome (percentage of sample). MD=Mean difference. CrI=credible interval. RR=risk ratio. AMI=amisulpride. ARI=aripiprazole. ASE=asenapine. BRE=brexpiprazole. CAR=cariprazine. CLO=clozapine. CPX=clopenthixol. CPZ=chlorpromazine. FLU=fluphenazine. FPX=flupentixol. HAL=haloperidol. ILO=iloperidone. LEV=levomepromazine. LOX=loxapine. LUR=lurasidone. MOL=molindone. OLA=olanzapine. PAL=paliperidone. PBO=placebo. PEN=penfluridol. PERA=perazine. PERPH=perphenazine. PIM=pimozide. QUE=quetiapine. RIS=risperidone. SER=sertindole. SUL=sulpiride. THIOR=thioridazine. THIOT=thiotixene. TRIFLU=trifluoperazine. ZIP=ziprasidone. ZOT=zotepine. ZUC=zuclopenthixol. *Results for clozapine and zotepine might be statistical artifacts caused by two small outlier studies.