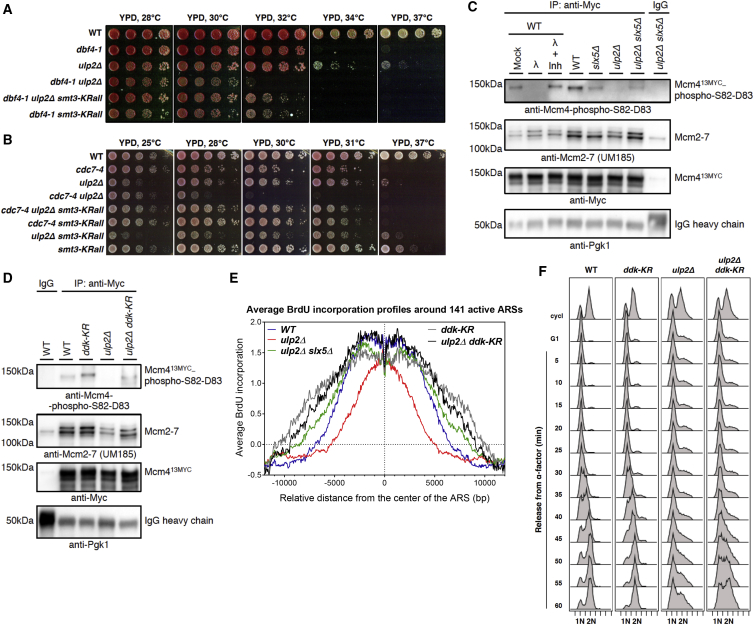
Figure 6.
Ulp2 Supports Efficient Replication Onset by Safeguarding MonoSUMOylated DDK Engaged in Replication
(A and B) Synthetic lethality of dbf4-1 ulp2Δ and cdc7-4 ulp2Δ cells at permissive temperatures for dbf4-1 and cdc7-4 single mutants is suppressed by a lysine-less SUMO variant (smt3-KRall).
(C) Reduced DDK-mediated Mcm4 phosphorylation in ulp2Δ cells is suppressed by deleting SLX5. Shown is IP of Mcm413MYC from exponentially growing WT, slx5Δ, ulp2Δ, and ulp2Δ slx5Δ cells. The specificity of the anti-Mcm4-phospho-S82-D83 antibody was evaluated by lambda phosphatase treatment (λ) with or without phosphatase inhibitors (Inh).
(D) The SUMOylation-defective ddk-KR mutant rescues reduced DDK-dependent Mcm4 phosphorylation in ulp2Δ cells; as in (C), but Mcm413MYC IP from WT, ddk-KR, ulp2Δ, and ulp2Δ ddk-KR cells.
(E) The decrease in BrdU incorporation in ulp2Δ cells is suppressed by ddk-KR, similar to slx5Δ. Shown is BrdU IP-on-chip analysis of cells released into S phase in the presence of 0.2 M HU and BrdU for 90 min after G1 arrest.
(F) The S phase progression defect in ulp2Δ cells is suppressed by ddk-KR. Exponentially growing WT, ddk-KR, ulp2Δ, and ulp2Δ ddk-KR cells (cycl) arrested in G1 phase by α-factor were released into yeast extract-peptone-dextrose (YPD) media at 25°C, and samples were taken every 5 min for FACS.
See also Figure S6.