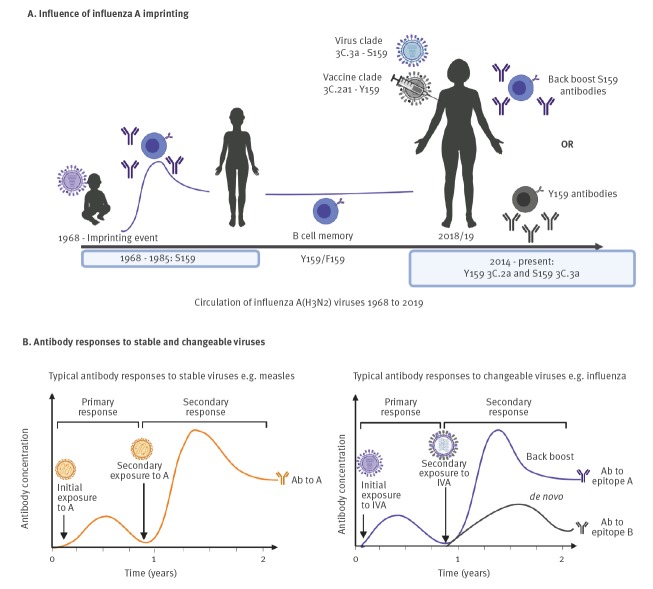
Figure 1.
Influenza imprinting, immune refinement, and imprint-regulated effect of vaccine (I-REV)
Ab: antibody; F: Phenylalanine; IVA: influenza virus A; S: Serine; Y: Tyrosine.
Panel A: Imprinting during infancy after the 1968 pandemic with an influenza A(H3N2) virus carrying a serine (S) at position 159 in the haemagglutinin antigenic B site may have led to antibodies elicited toward this position. During the 2018/19 season, the circulating 3C.3a viruses also carried a serine at amino acid 159. A re-exposure to S159 could back-boost previously acquired antibodies from the imprint. Concomitantly, the H3N2 vaccine component from the 2018/19 season was a 3C.2a1 virus with a tyrosine (Y) at 159 which was further exposed by a loss of glycosylation at site 160 following egg adaptation. Vaccination with the egg-adapted vaccine antigen may skew immune responses and antibody generation away from targeting epitopes needed for protection.
Panel B: At the second exposure of a stable virus such as measles virus, the antibody responses are boosted toward the original antigenic sites for a faster and larger response. In comparison, circulating influenza A(H3N2) viruses are constantly changing their antigenicity through antigenic drift. The viruses retain some antigenic similarity over time but changes also occur as a result. The secondary exposure of a person who has already been exposed to an influenza virus may lead to both back-boosting of originally acquired antibodies and also the development of antibodies to new epitopes.