Introduction
The relationship between cat exposure and allergic disease is complex. Having specific IgE Ab to cat has consistently been reported as a risk factor for wheezing.1-3 As a result, decreased exposure to cat allergens is recommended for cat allergic patients in treatment guidelines although there is very little controlled evidence that avoidance measures, including removing the cat are effective.4-6 The levels of cat allergen inhaled per day in a house with a cat are striking and have been estimated to be as high as 2 μg Fel d 1/day.7,8 This quantity is not much less than that used for sublingual desensitization, and is much higher than daily exposure calculated for pollen or dust mite allergens.8,9 Thus it is plausible that some of the skin test positive patients living in a house with a cat are “desensitized” as if they were on active immunotherapy.
Paradoxically despite the high levels of allergen exposure in the home, living with a cat is not consistently associated with an increased prevalence of sensitization to cat.10 Indeed in some studies, children raised in a house with a cat, or a dog, have been found to have a significantly lower prevalence of IgE to cat, or dog, allergens respectively.11-17 In addition to this, many of the non-sensitized children living in a house with a cat have been shown to have IgG and IgG4 Ab specific for Fel d 1.12,18 In contrast in a recent study of adults, acquisition of a cat was a risk factor for new onset cat sensitization.19 It has also been repeatedly observed that some college students experience either new, or increased symptoms after returning from college to a home with a cat that previously was not a problem. The changes in IgG and IgE Ab responses to cat allergen that can result from avoidance of cat allergen have not been previously studied.
The present study was designed to answer the question of how levels of serum IgG and IgE antibodies to cat allergens changed with the natural model of cat avoidance that occurs when students who had a cat in their family home moved to a residential college where pets were not allowed. In particular, we wanted to know whether there were contrasting changes in different groups of students which could help to explain why the results of allergen avoidance studies have been difficult to interpret.
Methods
During the fall of three consecutive years, 97 students attending college were recruited for a prospective cohort study after they responded to an internet advertisement. Participants were recruited based on cat ownership immediately prior to enrollment at the university in order to enrich the group for subjects who tolerated living in a house with a cat. The study was approved by the human investigation committee at the University of Virginia, and all participants provided written, informed consent.
At enrollment, participants completed an International Study of Asthma and Allergies in Childhood (ISAAC)-based questionnaire to assess symptoms of allergic disease. Additional questions about previous pet exposure were included. Skin prick allergy testing was performed with commercial cat extract at standardized allergen concentration (10,000 BAU/ml) and positive and negative controls (Greer, Lenoir, N.C.). Blood was collected for serum measurement of specific IgE Ab to Dermatophagoides pteronyssinus, cat, dog, cockroach, Aspergillus fumigatus, Alternaria alternata, oak, ryegrass, and ragweed (Phadia/Thermo Fisher Scientific, Uppsala, Sweden). Specific IgE Ab to Fel d 1 was measured using streptavidin CAPS and biotinylated Fel d 1.20 Serum IgG Ab to Fel d 1 was measured using radioimmunoprecipitation.12 In the sera with detectable IgG Ab to Fel d 1, Ab of the IgG4 isotype were measured using a mouse monoclonal Ab to human IgG4 followed by a goat anti-mouse IgG which had been absorbed over a human IgG sepharose column to remove cross reacting Ab.12
The primary study period for each student was one academic year. Visit one took place at the beginning of the academic year and the second visit was scheduled after students had experienced eight months of decreased exposure to cat allergen. All students were invited to follow up after the second year, but this was not an essential part of the study. At each visit, IgE and IgG Ab to cat were measured.
Cat allergen exposure was measured in a range of student dormitories (n=22). Local homes with (n=14) and without (n=12) cats were measured for comparison.21 Air samples were obtained using an ion charging device.8,21,22 Fluorescent multiplex array technology was used to measure cat allergen, Fel d 1 (Indoor Biotechnologies, Charlottesville, VA).23
Students were classified into three different immune groups based on their skin test and Ab responses to cat: sensitized, non-sensitized with IgG, and non-responders. Those having a positive skin test (wheal ≥3mm larger than the negative control) and/or specific IgE to cat ≥0.35 IU/ml were considered sensitized. The second group included students with cat specific IgG Ab without cat specific IgE or a positive skin test. Students with a negative skin test to cat and no significant specific IgE or IgG Ab response to cat were labeled as non-responders. Group characteristics at enrollment were compared using chi square. The primary outcome measure was the change in antibody titers over time. Measurements of IgE and IgG Ab were log transformed, and geometric mean titers (GMT) were compared longitudinally by paired t test. Airborne allergen measurements were log transformed for analysis. Geometric means (GM) were compared using independent t-test. Statistical tests were performed using SPSS 19 (IBM Corporation, Somers, New York).
Results
Group characteristics based on different immune responses to cat
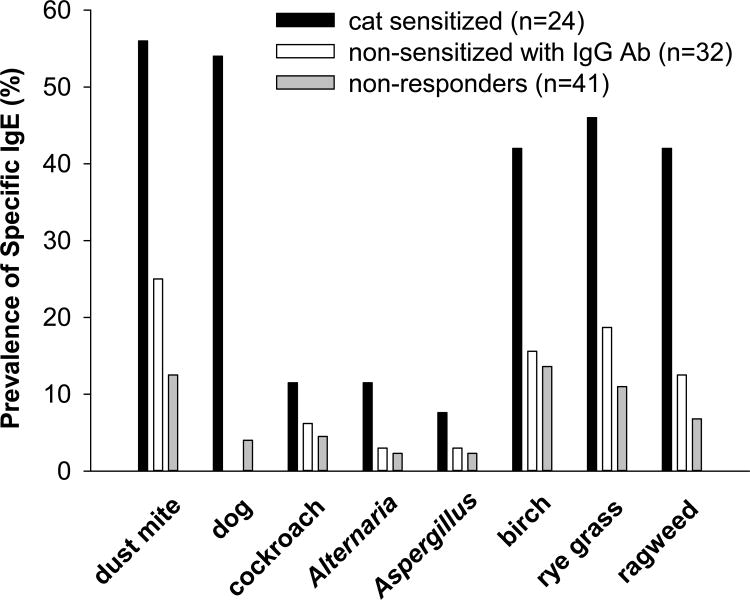
Among 97 students who had lived with a cat prior to college, having IgG without evidence of sensitization was the most frequently observed immune response to cat, n=32 (32.9%). Sensitization to cat was found in 24 students (24.7%) and 41 (42.2%) were labeled non-responders (Table 1). All sensitized students were skin prick test positive to cat except for one participant who was taking antihistamines. She had a negative skin prick test and a suppressed histamine response. Having specific IgE to other allergens was more frequent among students with sensitization to cat, and total IgE was significantly higher among cat sensitized students. However, nearly one third of the students showing IgG without IgE to cat, had evidence of sensitization to other allergens (Fig 1). None of the students who had IgG but were not sensitized to cat had wheezing (Table 1).
Table 1.
Characteristics of different subject groups as defined by antibody responses to cat allergen.
| Antibody responses to cat | ||||
|---|---|---|---|---|
|
|
||||
| Clinical feature | Sensitized (n=24) | Non-sensitized with IgG (n=32) | Non-responders (n=41) | P value* |
| Gender, n (% female) | 9 (37.5) | 26 (81.3) | 33 (80.5) | <0.001 |
| Wheezing†, n (%) | 11 (45.8) | 0 | 0 | nd‡ |
| Other sensitization, n (%)§ | 23 (95.8) | 10 (31.2) | 11 (26.8) | <0.001 |
| IgG4 Ab to Fel d 1, n (%) | 11 (45.8) | 11 (34.4) | ND | 0.8 |
| Lived in dormitory, n (%) | 23 (95.8) | 28 (87.5) | 39 (95.1) | 0.4 |
| Total IgE (IU/ml), GM (95% CI) | 184 (151-309) | 22.9 (20.1-61.1) | 24.8 (22.9-53.7) | <0.001± |
Tested by using Chi Square to compare between group differences except as indicated.
Wheezing is based on questionnaire response to “Have you had wheezing in the last 12 months?”
nd indicates not done.
“Other sensitization” is based on serum specific IgE measurements (see Fig 1).
Tested by using ANOVA to compare between group differences.
Fig 1.
The prevalence of having specific IgE to other inhalant allergens in students who are cat sensitized (black bar), IgG positive to Fel d 1 but non-sensitized to cat (white bar), and non-responders to cat allergen (gray bar).
Changes in antibody titers to Fel d 1 in non-sensitized subjects
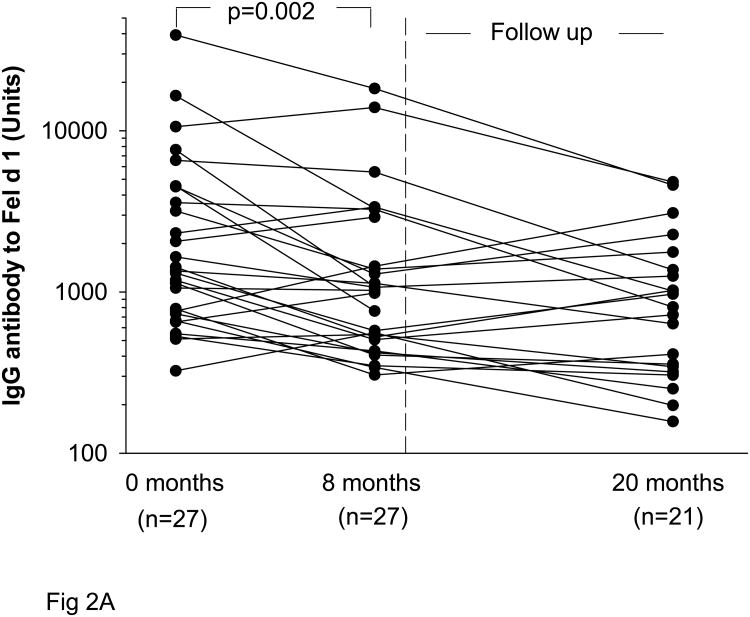
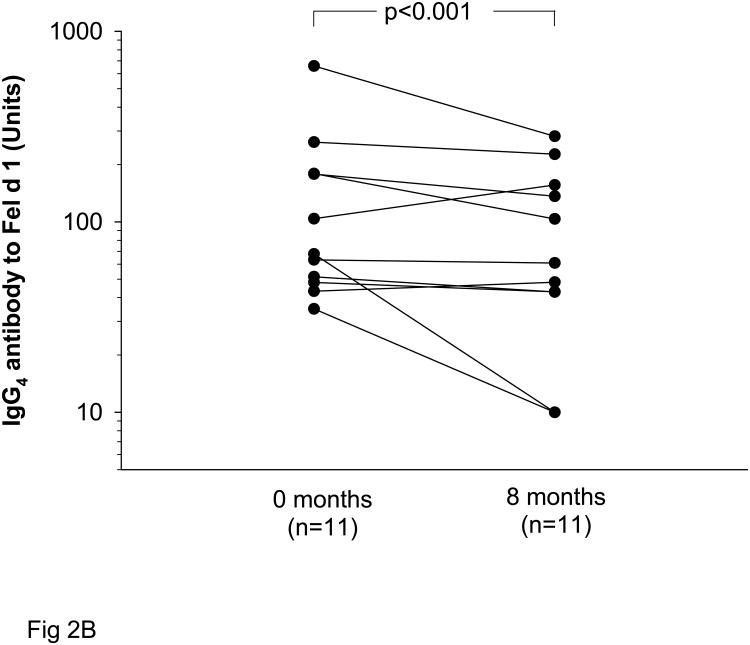
Non-sensitized subjects with IgG to Fel d 1 experienced a significant decline in IgG titers with decreased exposure to cat allergen. With decreased exposure to cats during the eight month study period, IgG Ab titers fell from GMT 1870 Units, 95% CI (1140-3050) to 1150 Units, 95% CI (955-2340) (p=0.002) (Fig 2A). For those students (n=21) who were followed for a second year, levels of IgG antibody to Fel d 1 had fallen further [GMT 790 Units, 95% CI (617-1550)] (Table 2). Among these non-sensitized subjects, IgG4 also decreased (p<0.001) during eight months of decreased cat exposure (Fig 2B). Of note, none of the individuals having IgG to Fel d 1 without sensitization to cat developed a positive serum IgE Ab assay or positive skin prick test to cat over one year (27 subjects) or two years (21 subjects).
Fig 2.
Titers of IgG (A) and IgG4 (B) to Fel d 1 among students who were non-sensitized to cat at the beginning of the academic year (0 months) and after eight months of decreased exposure to cat. Some students returned after a second year (20 months).
Table 2.
Changes in cat specific IgE and IgG antibody titers in three immunologically distinct groups of college students.
| STUDY PERIOD | FOLLOW UP | ||||||
|---|---|---|---|---|---|---|---|
| Enrollment | Visit One | Visit Two* | |||||
| 0 months | 8 months | 0 months† | 20 months | ||||
|
|
|
||||||
| mean | mean | p value | mean | mean | p value | ||
|
|
|||||||
| Sensitized | (n=24) | (n=20) | (n=20) | (n=17) | (n=17) | ||
| Skin prick test positive | 23/24 | 19/20 | 20/20 | 15/15 | |||
| IgE to cat (IU/ml) | 2.3 | 2.1 | 2.4 | 0.3 | 2.6 | 2.1 | 0.1 |
| IgE to Fel d 1 (IU/ml) | 2.1 | 1.8 | 1.5 | 0.08 | 1.6 | 1.2 | 0.2 |
| IgG to Fel d 1 (Units) | 2670 | 2820 | 1530 | <0.001 | 2790 | 1320 | 0.001 |
| Non-sensitized with IgG | (n=32) | (n=27) | (n=27) | (n=21) | (n=21) | ||
| Skin prick test positive | 0/32 | 0/27 | 0/27 | 0/21 | |||
| IgE to cat | <0.35 | <0.35 | <0.35 | <0.35 | |||
| IgE to Fel d 1 | <0.35 | <0.35 | nd | <0.35 | |||
| IgG to Fel d 1 | 1870 | 1870 | 1150 | 0.002 | 1620 | 790 | <0.001 |
| Non-responders | (n=41) | (n=21) | (n=21/25§) | (n=31) | |||
| Skin prick test positive | 0/41 | 0/21 | 0/21 | 0/31 | |||
| IgE to cat | <0.35 | <0.35 | <0.35 | <0.35 | |||
| IgE to Fel d 1 | <0.35 | <0.35 | nd | <0.35 | |||
| IgG to Fel d 1 | <50 | <50 | <50 | <50 | |||
All statistics for Visit Two are calculated on data from students who completed both visits.
Geometric mean titers at enrollment for only the subjects who presented for the follow up visit (20 months) to show the total change in antibody titers in those individuals.
nd is not done.
Twenty-five non-responders were invited for a second study visit after eight months of avoidance; 21 returned for testing. All 41 non-responders in the study were invited for follow up after 20 months to determine whether antibody responses in these subjects had changed over an extended period of time. An additional 14 returned so that 35 non-responders were monitored for at least one year in the study.
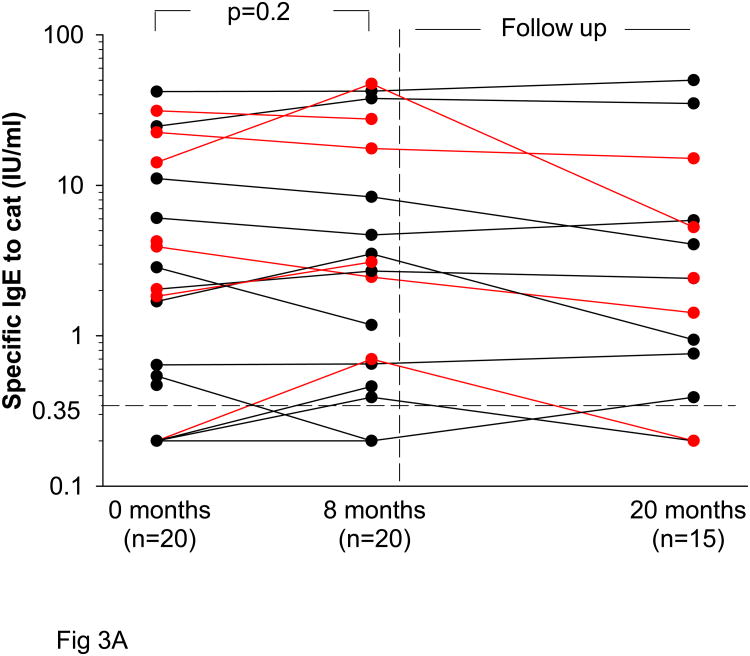
Antibody changes in cat sensitized subjects
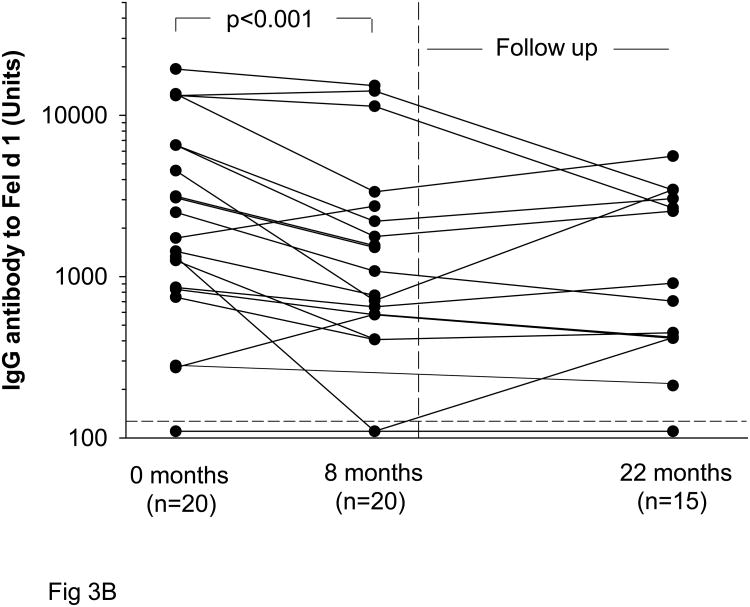
Specific IgE titers to cat did not decrease significantly during prolonged, decreased exposure to cat allergen (Fig 3A). By contrast similar to the non-sensitized subjects, IgG Ab to Fel d 1 decreased significantly during the eight month study period from GMT 2820 Units, 95% CI (2160-6930) to 1530 Units, 95% CI (1120-3800) (p<0.001) (Fig 3B). In sensitized students who returned for follow up during the second year, GMT 1320 Units, 95% CI (890-3020) was measured (Table 2).
Fig 3.
Levels of cat specific IgE (A) at the beginning of the academic year (0 months) and after eight months of decreased exposure to cat. Some students returned after a second year (20 months). Students with wheezing are shown in red. Titers of IgG to Fel d 1 (B) in cat sensitized students.
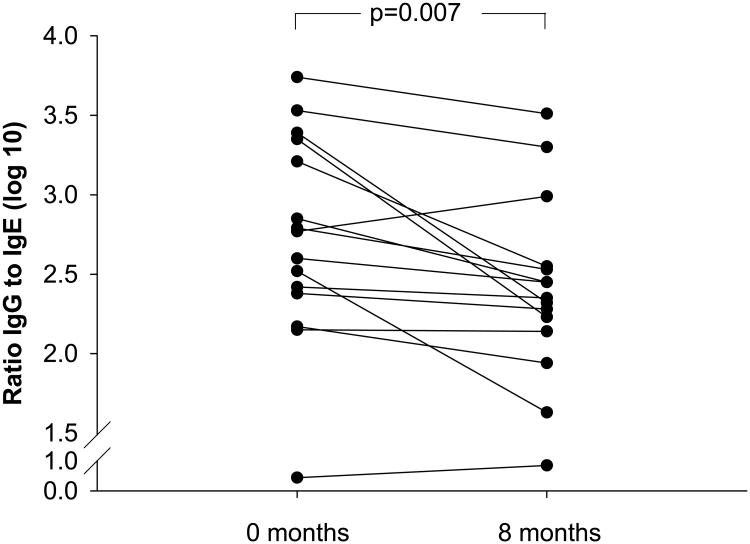
We also analyzed changes in the ratio of IgG to IgE Ab (Fig 4) during the study. In keeping with the changes in IgG shown previously, there was a highly significant decrease in the ratio of IgG to IgE Ab. In fact, ratios decreased in all but three subjects over the first eight months of decreased exposure (p=0.007), and in a few individuals, the decreases were quite dramatic. We show results for three cases that illustrate a large change in ratios and a parallel fall in IgG4 (eFig 1).
Fig 4.
Ratios of cat specific IgG to IgE Ab were calculated at the beginning of the study and after eight months of marked, decreased exposure to cat allergen.
Exposure to cat allergen
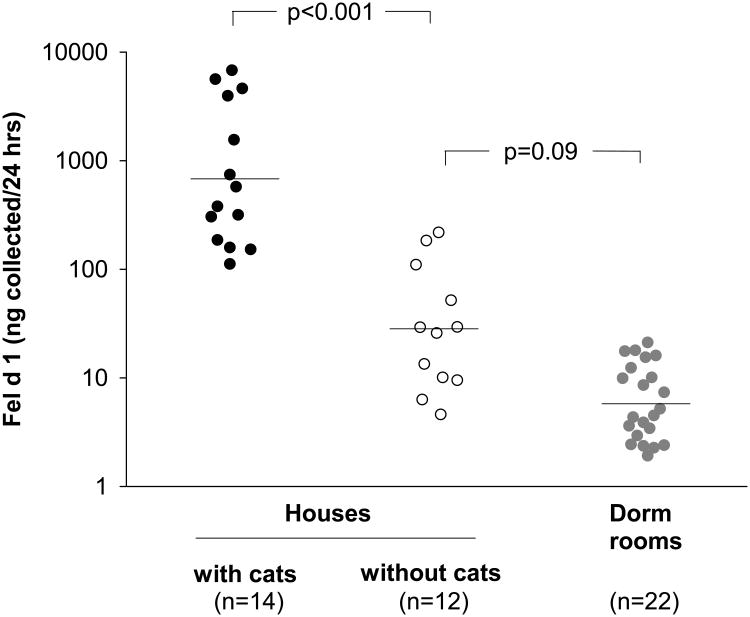
In our cohort, all students lived without a cat during the first year of the study. Specifically, 90 students (92.8%) lived in a dormitory and the remainder lived in student apartments (see Table 1). In representative student dormitories without cats, low levels of the cat allergen Fel d 1 were measurable in airborne samples (Fig 5). We found that dormitory exposure levels (GM 6 ng/24 hrs) were 100 times lower than levels found in Charlottesville homes with a cat (GM 700 ng/24 hrs) and ∼5 times lower than levels found in homes without a cat (GM 30 ng/24 hrs).
Fig 5.
Airborne Fel d 1 was measured by using an ion charging device in three different environments. Horizontal lines depict geometric mean levels. Allergen collected over 24 hours is shown. Using a flow rate of 1.3 m3/min and collection efficiency of 45%, the estimated sampling rate to calculate airborne concentration is 0.6 m3/min.8
Discussion
After moving to a university residential hall, individuals who come from a home with a cat experience a major decrease in exposure to cat allergens. Using this natural model of avoidance of cat allergens we have monitored IgG and IgE Ab levels over time. While individual variation was observed and cat exposure undoubtedly varied, overall IgG Ab titers to Fel d 1 fell significantly during the first 8 months of avoidance, while IgE Ab did not decrease. Among sensitized students we saw a major decrease in the ratio of IgG to IgE Ab. There were 56 students who had IgG Ab to Fel d 1 alone or had no measurable response to cat, who were followed for at least two years at college. All these students were initially skin prick test negative to cat and none of them developed positive skin prick tests to cat allergen or serum IgE Ab to either cat allergen or Fel d 1.
Within this study, there were three groups of students who did not report symptoms related to cats. There were two groups of skin test negative subjects, those with and without IgG Ab to Fel d 1. There were also skin test positive individuals, who prior to coming to university, lived in a house with a cat and did not appear to have allergic symptoms or asthma related to the cat. Clearly, similar skin test positive but non-symptomatic individuals are seen in relation to many allergens. However, very few other allergens involve levels of allergen exposure comparable to living in a house with a cat. This phenomenon of having positive skin prick tests to pet dander without symptoms of allergic disease has recently been reported in children who live in homes with a dog as well.3
While the mechanisms of the observed changes in the immune response to cat are not clear, these changes are not consistent with some proposed models of tolerance. Increased exposure to the microbial cell wall component, endotoxin, is one of the theories proposed to explain the effect of pet ownership on allergic responses.16 From a simplistic standpoint, endotoxin exposure could protect against allergic sensitization by deviating the immune response from a Th2-type predominated by production of the cytokine IL-4 and IL-13 to a Th1-type with increased production of IFN-γ.24 This type of immune deviation has been reported in farming communities where high levels of endotoxin and/or bacterial exposure have been associated with a decreased risk of atopy and asthma.25,26 If tolerance to cat resulted from a shift to Th1 immunity, induced by endotoxin on cat dander particles, we would not expect IgG4 Ab as part of the response. Other investigators have also found the effect of endotoxin to be distinct from that of pets.27 Furthermore increased levels of airborne endotoxin have not been found in homes with cats.21,28
The fact that IgG Ab levels fell during the time these students were in college is in keeping with some recently proposed models of the control of IgE Ab production. In 2004, Aalberse and Platts-Mills proposed that low levels of ubiquitous cat allergen exposure could sustain IgE production by long lived plasma cells in the bone marrow.29,30 In contrast, IgG production would decrease with the lower levels of antigen stimulation and increase upon re-exposure consistent with the priming of B cells in germinal centers and the production of IgG and IgG4 by plasma cells in local lymph nodes.29 Although studies on “blocking” or IgG Ab date back more than 70 years, there is still confusing evidence about whether IgG Ab are directly protective. The problem is that IgG Ab may simply reflect high exposure either natural or from immunotherapy, and that there may be parallel changes occurring in other aspects of the immune or inflammatory responses to allergen exposure. This raises the question of whether the lack of symptoms among a significant proportion of the cat sensitized individuals living in a house with a cat represents another form of tolerance. Some bee keepers develop clinical tolerance during the season when they usually receive multiple stings.31 Good evidence has been presented that this involves increased IL-10 production by T cells and an increase in IgG and/or IgG4 Ab relative to IgE Ab.31,32 That response is known to be transient returning close to initial levels within months of the end of the bee keeping season. Thus, we would propose that subjects living with a cat are at least partially desensitized by the high levels of natural exposure. While the present study does not address symptoms, there is evidence from other forms of inhalant allergy that subjects with high levels of IgG and IgG4 Ab report fewer symptoms.33,34 Also in keeping with this model, we have reported high levels of IL-10 production by T cells of subjects with a “modified Th2” response.35
On a larger scale when considering allergic diseases in general, these results emphasize that recommendations for allergen avoidance are not straightforward. Our understanding of individual responses to cat avoidance has already been complicated by studies showing decreased sensitization to cats, and in some studies decreased asthma, among children living with a cat at home.11,12,15,16 Previously, the simplest view was that avoidance should be recommended only for patients who were skin test positive to cat. However, since many of these students are allergic to more than one allergen it may not be correct to simply to assume that symptoms among skin test positive individuals living in a house with a cat are due to cat exposure. Now, it seems possible that some of those subjects with IgE Ab who have a major decrease in IgG Ab could develop increased symptoms on re-exposure after giving up their pets. Similar observations have been made in regards to food allergy.36 Specifically, investigators who studied cow's milk allergy among children with eczema, reported that many who had previously tolerated milk without acute reactions experienced immediate symptoms with accidental exposure after prolonged avoidance (median 2.3 years).36
There are several limitations to our study. First, based on our own measurements in college dormitories and on other reports of measurable cat allergen in public places, we know that this is not a study of complete cat allergen avoidance. We believe the results of the study are important because they represent the reality facing our patients. Even those who choose to remove a cat from their home cannot expect to avoid exposure in the community.37-39 In all previous studies of allergen avoidance the patients have been free to visit other homes or public places where allergen exposure can also occur. Primarily the present study was designed to follow antibody responses to cat during a period of decreased exposure. We designed this study to follow immune responses in order to obtain information about which immunological group would have changes that could result in new or increased symptoms with avoidance. There were no consistent reports of change in symptoms on returning home. However, it was clear that these responses were confounded by several factors i) the students were going to many different houses and vacation sites, most of which were too far from Charlottesville to be studied; ii) it is well established that there can be wide variation in cat allergen measurements in homes with or without a cat; and iii) many of the students were sensitized to more than one allergen (96% of the cat sensitized group) so that it was difficult to interpret whether their symptoms were related to cat exposure. For these reasons, we believe it would be difficult to adequately evaluate any change in symptoms upon return to home environments. Based on our present results, we estimate that answering questions about changes in symptoms would require following at least 100 skin test positive individuals for one year in college.
In conclusion, a major question in our original plan was whether some of the students with IgG and IgG4 Ab, but without any evidence of sensitization, would develop IgE Ab or positive skin prick tests specific for cat during their first year in college. We did not observe any cases where skin tests became positive. By contrast, there were consistent decreases in IgG Ab; and among the skin test positive individuals, there were major changes in the ratio of IgG to IgE. Thus, increases in symptoms upon returning home could be due to the changes in antibody levels observed in skin test positive subjects. While a lack of symptoms is frequently observed in individuals with positive skin tests to other allergens, the interesting thing about the individuals with IgE Ab to cat is that they tolerate living with high levels of airborne exposure. The results suggest that decisions about avoidance advice in relation to cats, and probably other domestic animals, should not make simple assumptions about the effects of decreased exposure. Furthermore, it seems that future studies of avoidance would be most useful if they considered skin test positive subjects with and without symptoms.
Supplementary Material
eFig 1: Changes in titers of cat specific IgE are shown with changes in IgG and IgG4 to Fel d 1 over eight months of decreased exposure in three sensitized subjects, no. 1042 (A), no. 1073 (B), and no. 1015 (C).
Acknowledgments
Funding: National Institutes of Health grants K23AI-059317, R01 AI-20565 and AI-AADCRC-U19-070364
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sears MR, Herbison GP, Holdaway MD, Hewitt CJ, Flannery EM, Silva PA. The relative risks of sensitivity to grass pollen, house dust mite and cat dander in the development of childhood asthma. Clin Exp Allergy. 1989;19:419–24. doi: 10.1111/j.1365-2222.1989.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 2.Call RS, Smith TF, Morris E, Chapman MD, Platts-Mills TAE. Risk factors for asthma in inner city children. J Pediatr. 1992;121:862–6. doi: 10.1016/s0022-3476(05)80329-4. [DOI] [PubMed] [Google Scholar]
- 3.Stoltz DJ, Jackson DJ, Evans MD, et al. Specific patterns of allergic sensitization in early childhood and asthma and rhinitis risk. Clin Exp Allergy. 2013;43:233–41. doi: 10.1111/cea.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood RA, Chapman MD, Adkinson NF, Jr, Eggleston PA. The effect of cat removal on allergen content in household-dust samples. J Allergy Clin Immunol. 1989;83:730–4. doi: 10.1016/0091-6749(89)90006-7. [DOI] [PubMed] [Google Scholar]
- 5.Boulet LP. Approach to Adults with asthma. In: Adkinson NF, Bochner BS, Busse WW, Holgate ST, Lemanske RF, Simons FER, editors. Middleton's Allergy Principles and Practice. 7th. Elsevier; 2009. pp. 1353–60. [Google Scholar]
- 6.Portnoy JM, Kennedy K, Sublett JL, Phipatanakul W, Matsui E, Barnes C. Environmental assessment and exposure control: a practice parameter furry-animals. Ann Allergy Asthma Immunol. 2012;108:223, e1–15. doi: 10.1016/j.anai.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Custovic A, Simpson B, Simpson A, Hallam C, Craven M, Woodcock A. Relationship between mite, cat, and dog allergens in reservoir dust and ambient air. Allergy. 1999;54:612–6. doi: 10.1034/j.1398-9995.1999.00062.x. [DOI] [PubMed] [Google Scholar]
- 8.Custis NJ, Woodfolk JA, Vaughan JW, Platts-Mills TAE. Quantitative measurement of airborne allergens from dust mites, dogs, and cats using an ion-charging device. Clin Exp Allergy. 2003;33:986–91. doi: 10.1046/j.1365-2222.2003.01706.x. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez-Cuesta E, Berges-Gimeno P, Gonzalez-Mancebo E, Fernandez-Caldas E, Cuesta-Herranz J, Casanovas M. Sublingual immunotherapy with a standardized cat dander extract: evaluation of efficacy in a double blind placebo controlled study. Allergy. 2007;62:810–7. doi: 10.1111/j.1398-9995.2007.01365.x. [DOI] [PubMed] [Google Scholar]
- 10.Lodrup Carlsen KC, Roll S, Carlsen KH, et al. Does pet ownership in infancy lead to asthma or allergy at school age? Pooled analysis of individual participant data from 11 European birth cohorts. PLoS One. 2012;7:e43214. doi: 10.1371/journal.pone.0043214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hesselmar B, Aberg N, Aberg B, Eriksson B, Bjorksten B. Does early exposure to cat or dog protect against later allergy development? Clin Exp Allergy. 1999;29:611–7. doi: 10.1046/j.1365-2222.1999.00534.x. [DOI] [PubMed] [Google Scholar]
- 12.Platts-Mills TAE, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitization, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–6. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 13.Perzanowski MS, Ronmark E, Platts-Mills TAE, Lundback B. Effect of cat and dog ownership on sensitization and development of asthma among preteenage children. Am J Respir Crit Care Med. 2002;166:696–702. doi: 10.1164/rccm.2201035. [DOI] [PubMed] [Google Scholar]
- 14.Custovic A, Hallam CL, Simpson BM, Craven M, Simpson A, Woodcock A. Decreased prevalence of sensitization to cats with high exposure to cat allergen. J Allergy Clin Immunol. 2001;108:537–9. doi: 10.1067/mai.2001.118599. [DOI] [PubMed] [Google Scholar]
- 15.Erwin EA, Wickens K, Custis NJ, et al. Cat and dust mite sensitivity and tolerance in relation to wheezing among children with high exposure to allergens. J Allergy Clin Immunol. 2005;115:74–9. doi: 10.1016/j.jaci.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 16.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288:963–72. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 17.Wegienka G, Johnson CC, Havstad S, Ownby DR, Nicholas C, Zoratti EM. Lifetime dog and cat exposure and dog- and cat-specific sensitization at age 18 years. Clin Exp Allergy. 2011;41:979–86. doi: 10.1111/j.1365-2222.2011.03747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Platts-Mills TAE, Perzanowski M, Woodfolk JA, Lundback B. Relevance of early or current pet ownership to the prevalence of allergic disease. Clin Exp Allergy. 2002;32:335–8. doi: 10.1046/j.1365-2222.2002.01352.x. [DOI] [PubMed] [Google Scholar]
- 19.Olivieri M, Zock JP, Accordini, et al. Risk factors for new-onset cat sensitization among adults: a population-based international cohort study. J Allergy Clin Immunol. 2012;129:420–5. doi: 10.1016/j.jaci.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 20.Erwin EA, Custis NJ, Satinover SM, et al. Quantitative measurement of IgE antibodies to purified allergens using streptavidin linked to a high-capacity solid phase. J Allergy Clin Immunol. 2005;115:1029–35. doi: 10.1016/j.jaci.2004.12.1131. [DOI] [PubMed] [Google Scholar]
- 21.Platts-Mills JA, Custis NJ, Woodfolk JA, Platts-Mills TAE. Airborne endotoxin in homes with domestic animal: implications for cat-specific tolerance. J Allergy Clin Immunol. 2005;116:384–9. doi: 10.1016/j.jaci.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Luczynska CM, Li Y, Chapman MD, Platts-Mills TAE. Airborne concentrations and particle size distribution of allergen derived from domestic cats (Felis domesticus) Am Rev Respir Dis. 1990;141:361–7. doi: 10.1164/ajrccm/141.2.361. [DOI] [PubMed] [Google Scholar]
- 23.Earle CD, King EM, Tsay A, et al. High-throughput fluorescent multiplex array for indoor allergen exposure assessment. J Allergy Clin Immunol. 2007;119:428–33. doi: 10.1016/j.jaci.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Gereda JE, Leung DY, Thatayatikom A, et al. Relation between house-dust endotoxin exposure, type 1 T-cell development, and allergen sensitisation in infants at high risk of asthma. Lancet. 2000;355:1680–3. doi: 10.1016/s0140-6736(00)02239-x. [DOI] [PubMed] [Google Scholar]
- 25.Braun-Fahrlander C, Riedler J, Herz U, et al. Environmental exposure to endotoxin and its relation to asthma in school-age chidren. New Engl J Med. 2002;347:869–77. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 26.Ege MJ, Mayer M, Normand A, et al. Exposure to environmental microorganisms and childhood asthma. New Engl J Med. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 27.Litonjua AA, Milton DK, Celedon JC, Ryan L, Weiss ST, Gold DR. A longitudinal analysis of wheezing in young children: the independent effects of early life exposure to house dust endotoxin, allergens, and pets. J Allergy Clin Immunol. 2002;110:736–42. doi: 10.1067/mai.2002.128948. [DOI] [PubMed] [Google Scholar]
- 28.Sohy C, Lieutier-Colas F, Casset A, et al. Dust and airborne endotoxin exposure in dwellings in the Strasbourg metropolitan area (France) Allergy. 2005;60:541–2. doi: 10.1111/j.1398-9995.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 29.Aalberse RC, Platts-Mills TAE. How do we avoid developing allergy: modifications of the TH2 response from a B-cell perspective. J Allergy Clin Immunol. 2004;113:983–6. doi: 10.1016/j.jaci.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 30.Davies JM, Platts-Mills TAE, Aalberse RC. The enigma of IgE(+) B-cell memory in human subjects. J Allergy Clin Immunol. 2013;131:972–6. doi: 10.1016/j.jaci.2012.12.1569. [DOI] [PubMed] [Google Scholar]
- 31.Meiler F, Zumkehr J, Klunker S, Ruckert B, Akdis CA, Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J Exp Med. 2008;205:2887–98. doi: 10.1084/jem.20080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meiler F, Klunker S, Zimmermann M, Akdis CA, Akdis M. Distinct regulation of IgE, IgG4, and IgA by T regulatory cells and toll-like receptors. Allergy. 2008;63:1455–63. doi: 10.1111/j.1398-9995.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 33.Jeal H, Draper A, Harris J, Taylor AN, Cullinana P, Jones M. Modified Th2 responses at high-dose exposures to allergen: using an occupational model. Am J Respir Crit Care Med. 2006;174:21–5. doi: 10.1164/rccm.200506-964OC. [DOI] [PubMed] [Google Scholar]
- 34.Jakobsen CG, Bodtger U, Poulsen LK, Roggen EL. Vaccination for birch pollen allergy: comparison of the affinities of specific immunoglobulins E, G1 and G4 measured by surface plasmon resonance. Clin Exp Allergy. 2005;35:193–8. doi: 10.1111/j.1365-2222.2005.02160.x. [DOI] [PubMed] [Google Scholar]
- 35.Reefer AJ, Carneiro RM, Custis NJ, et al. A role for IL-10-mediated HLA-DR7-restricted T cell-dependent events in development of the modified Th2 response to cat allergen. J Immunol. 2004;172:2763–72. doi: 10.4049/jimmunol.172.5.2763. [DOI] [PubMed] [Google Scholar]
- 36.Flinterman AE, Knulst AC, Meijer Y, Bruijnzeel-Koomen CAFM, Pasmans SGMA. Acute allergic reactions in children with AEDS after prolonged cow's milk elimination diets. Allergy. 2006;61:370–4. doi: 10.1111/j.1398-9995.2006.01018.x. [DOI] [PubMed] [Google Scholar]
- 37.Sporik R, Ingram JM, Price W, Sussman JH, Honsinger JW, Platts-Mills TAE. Association of asthma with serum IgE and skin test reactivity to allergens among children living at high altitude Tickling the dragon's breath. Am J Respir Crit Care Med. 1995;151:1388–92. doi: 10.1164/ajrccm.151.5.7735590. [DOI] [PubMed] [Google Scholar]
- 38.Perzanowski MS, Rönmark E, Nold B, Lundbäck B, Platts-Mills TAE. Relevance of allergens from cats and dogs to asthma in the northernmost province of Sweden: schools as a major site of exposure. J Allergy Clin Immunol. 1999;103:1018–24. doi: 10.1016/s0091-6749(99)70173-9. [DOI] [PubMed] [Google Scholar]
- 39.Almqvist C, Wickman M, Perfetti L, et al. Worsening of asthma in children allergic to cats, after indirect exposure to cat at school. Am J Respir Crit Care Med. 2001;163:694–8. doi: 10.1164/ajrccm.163.3.2006114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFig 1: Changes in titers of cat specific IgE are shown with changes in IgG and IgG4 to Fel d 1 over eight months of decreased exposure in three sensitized subjects, no. 1042 (A), no. 1073 (B), and no. 1015 (C).