Fig. 2. TNFα programming maintains DC1 programming and TH1 priming in response to immune skewing by immunomodulatory antigen.
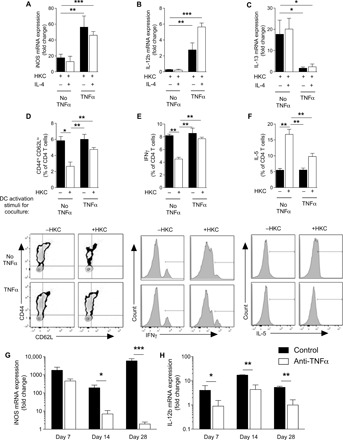
DC1-polarized BMDCs generated by IFNγ in the presence (TNFα) or absence (No TNFα) of supplemental TNFα were treated with heat-killed C. neo (HKC). (A and B) DC1 (iNOS and IL-12b) and (C) DC2 (IL-13) genes from BMDCs were quantified by qPCR. *P < 0.05, **P < 0.01, and ***P < 0.001 between indicated sample and “HKC-treated No TNFα” sample. Note that there were no significant differences between HKC-treated No TNFα samples regardless of IL-4 challenge. n = 18 from three separate matched experiments. (D to F) BMDCs generated from OT-II mice were stimulated to become DC1 in the presence (TNFα) or absence (No TNFα) of TNFα, and HKC were incubated with naïve splenic CD4 T cells and OVA peptide for 5 days. (D) CD4 T cell activation (CD44hiCD62Llo) and frequency of (E) IFNγ-producing and (F) IL-5–producing T cells were assessed by flow cytometry as seen in representative flow plots and cumulative data. n = 6. *P < 0.05, **P < 0.01, and ***P < 0.001 between the indicated sample and HKC-treated No TNFα sample. (G and H) Magnetically sorted CD11c+ cells isolated from mouse lung at 7 and 14 dpi and qPCR performed for iNOS (G) and IL-12b (H). n = 8 per group from two separate matched experiments conducted months apart and from different cages. *P < 0.05, **P < 0.01, and ***P < 0.001 between the indicated samples.
