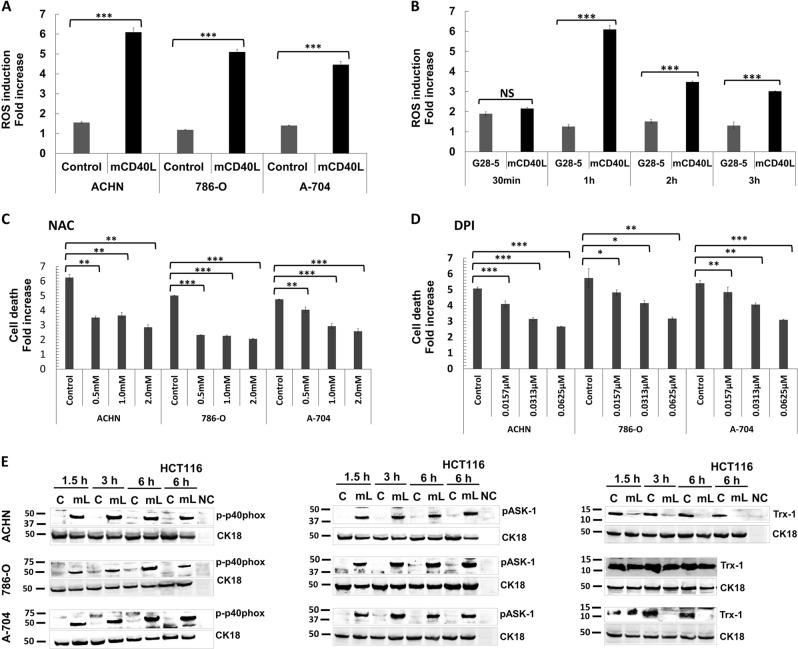
Fig. 8. ROS generation by mCD40L, but not soluble CD40 agonist, and activation of ROS-dependent signalling for apoptosis induction.
a ACHN, 786-O and A-704 cells were co-cultured with 3T3Neo or 3T3CD40L effector cells for 1 h and intracellular ROS was detected by treatment with 1 μM H2DCFDA (see Methods). Background-corrected relative fluorescence unit (RFU) readings obtained were used to present the data as ROS induction fold increase, which is fold change in RFU detected for H2DCFDA-treated 3T3Neo/RCC cell (‘Control’) and 3T3CD40L/RCC cell (‘mCD40L’) co-cultures vs. untreated co-cultures. Results are representative of three independent experiments. Bars show mean fold change of 5–6 technical replicates ± SEM. b To compare the differences in the extent of ROS generation for mCD40L vs. soluble agonist, ACHN cells were treated with ‘mCD40L’ (alongside negative controls) or agonistic anti-CD40 mAb (‘G28-5′) for the indicated time periods (30 min, 1 h, 2 h and 3 h) and ROS levels were detected as in a. For mCD40L treatments, data are presented as background-corrected RFU readings of mCD40L relative to controls; for G28-5 treatments, data are presented as RFU readings relative to untreated cells. Results (ROS induction fold increase) are representative of two independent experiments. Bars show mean fold change of 4–5 technical replicates ± SEM. c, d ACHN, 786-O and A-704 cells were treated with mCD40L in the absence (vehicle control—denoted ‘Control’) or presence of the indicated concentration of inhibitors NAC (c) and DPI (d). Cell death was detected 48 h later using the CytoTox-Glo assay (see Methods). Results are presented as Cell death fold increase in background-corrected RLU readings relative to control (mCD40L treatment vs. controls) and are representative of three independent experiments. Bars show mean fold change of 4–5 technical replicates ± SEM. e ACHN, 786-O and A-704 cells were treated with mCD40L for the indicated time periods (1.5, 3 and 6 h) and expression of phospho-p40phox, phospho-ASK1 and Trx-1 was detected in controls (‘C’) vs. mCD40L-treated cells (‘mL’) by immunoblotting (40 µg protein/lane). Equal loading for human epithelial cell lysate was confirmed by CK18 detection (see Methods). As positive controls, lysates from HCT116 cells that were treated with control (‘C’) or treated with mCD40L (‘mL’) for 6 h were included. Lysate from effector (3T3CD40L) cell monocultures served as negative control (NC) and confirmed the human-protein specificity of the antibodies.