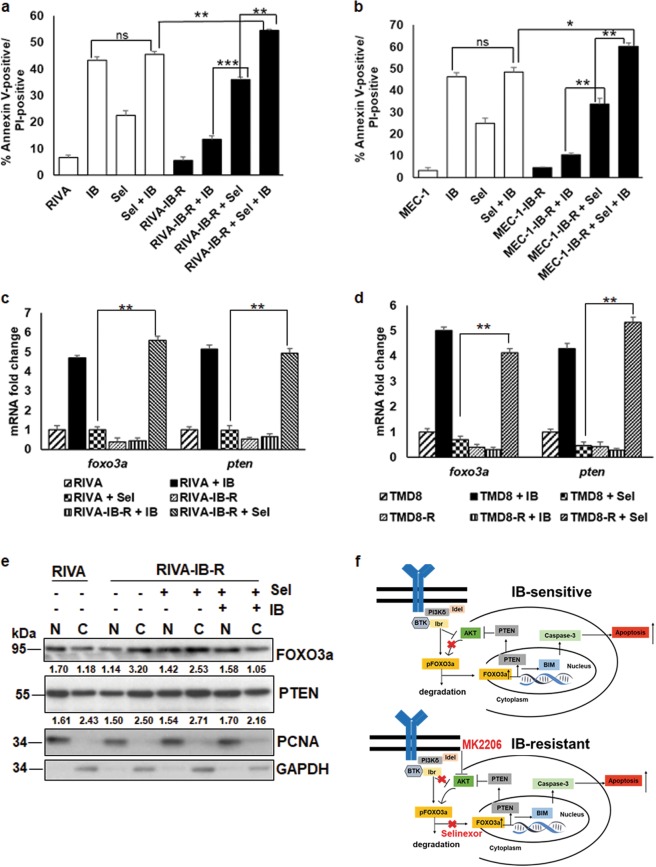
Fig. 6. Selinexor synergizes with ibrutinib by restoring FOXO3a nuclear accumulation in IB-R cells.
a, b RIVA/ RIVA-IB-R and MEC-1/ MEC-1-IB-R cells were treated with ibrutinib and selinexor either alone or in combination for 24 h. Cell viability was determined by Annexin V-PI staining. Control cells were treated with DMSO. (*p < 0.05, **p < 0.01, ***p < 0.001). SD is indicated as error bars (N = 3). c, d mRNA fold change of foxo3a and pten in parental vs IB-R RIVA and TMD8 cells treated with either ibrutinib or selinexor (0.5 µM). e RIVA-IB-R cells were treated with selinexor (500 nM) and ibrutinib (1 µM) alone or in combination. Expression levels of FOXO3a and PTEN were examined in nuclear and cytoplasmic fractions. PCNA and GAPDH were used as loading controls for the nuclear and cytoplasmic fractions, respectively. First two lanes indicate baseline expression of proteins in RIVA cells. Model of acquired IB-R mechanism. f Reduced FOXO3a phosphorylation (FOXO3aSer253) followed by its nuclear accumulation after ibrutinib treatment increases transcriptional activation of PTEN and BIM, leading to apoptosis and AKT inactivation in IB-sensitive cells (upper panel). In IB-R cells, pFOXO3aSer253 is upregulated resulting into its reduced nuclear accumulation and transcriptional inhibition of PTEN and BIM. Selinexor overcomes IB-R by restoring nuclear accumulation of FOXO3a leading to transcriptional activation of PTEN and BIM thus, leading to apoptosis. Idelalisib and MK2206 overcome IB-R by exploiting IB-R cells’ dependency on PI3K/AKT pro-survival signaling (lower panel).