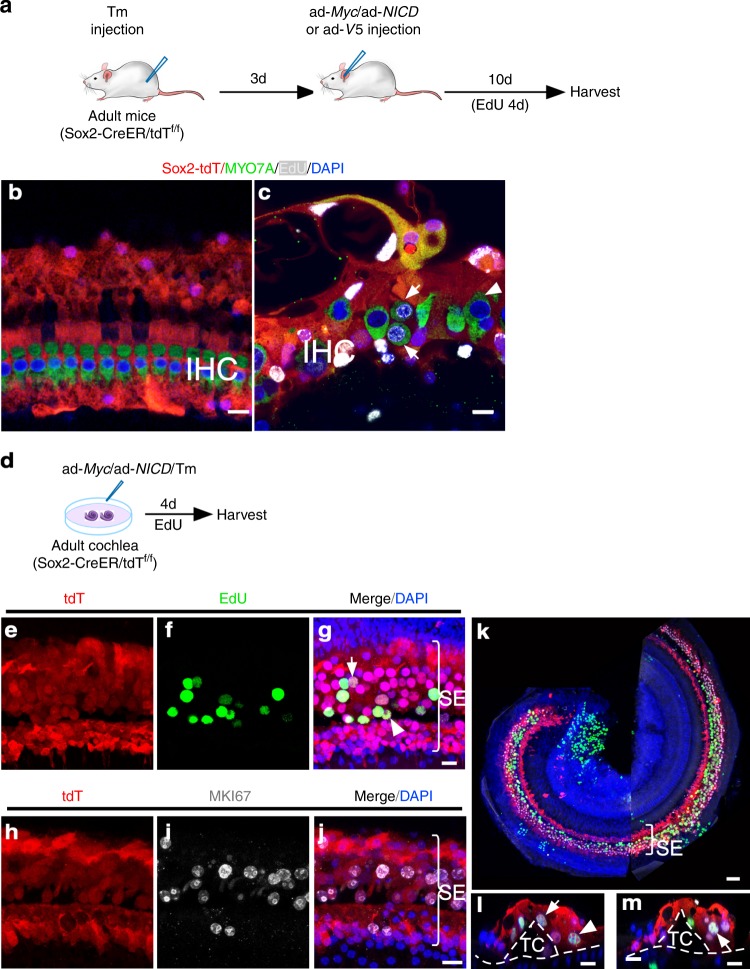
Fig. 3.
Study of the origin of dividing HCs and SCs in adult cochlea by lineage tracing. a A schematic diagram depicting the in vivo experimental procedure. b In control adult Sox2-CreER/tdTf/f mice 10 days after ad-V5 injection and Tamoxifen (Tm) treatment, all IHCs were tdT negative and were surrounded by tdT+ SCs. No EdU labeling was detected. c Following Tm treatment and ad-Myc/ad-NICD injection into adult Sox2-CreER/tdTf/f mouse cochlea for 10 days, dividing IHCs were detected (arrows). All IHCs (dividing, arrows; non-dividing, arrowhead) were tdT negative. d Schematic diagram of lineage-tracing of proliferating adult SCs in vitro. e–g EdU labeling in the SCs (tdT+) in cultured adult Sox2-CreER/tdTf/f cochlea after treatment with Tm and infection with ad-Myc/ad-NICD. Judging by their locations, likely dividing Deiters’ cells (arrow) and pillar cells (arrowhead) were identified. Bracket indicates the sensory epithelium (SE). h–j MKI67 labeling in the SCs (tdT+) in cultured adult Sox2-CreER/tdTf/f cochlea after treatment with Tm and infection with ad-Myc/ad-NICD. k A confocal image of the surface view of the middle turn of adult Sox2-CreER/tdTf/f cochlea after treatment with Tm and infection by ad-Myc/ad-NICD to show numerous proliferating SCs (tdT+/EdU+) in the SE region. l, m z-axis cross-section views of confocal images in (k) showed dividing SCs by triple labeling of tdT+/EdU+/SOX2+ (likely Deiters’ cells, arrows; Hensen cell, arrowhead. tdT: red; EdU: green; SOX2: white). Dashed lines mark the basilar membrane and the tunnel of Corti (TC). Scale bars: 50 μm in (k); 10 μm in other figures.