Abstract
Seasonal variations in photoperiod are associated with alterations in human mood and behavior. Similarly, manipulation of the environmental lighting regimen can exert pronounced effects on affective behavior in experimental animals. These observations may be due, in part, to light-induced alterations in circadian rhythms, but it seems likely that other, non-circadian factors also contribute. Several studies have shown that voluntary alcohol (ethanol) consumption can be affected by lighting conditions in rodents, suggesting that photoperiodic variation may account for seasonal and geographic patterns of human alcohol consumption. Nevertheless, the existing animal data are somewhat inconsistent, and little work in this area has been performed in mice. In the present study, we monitored circadian activity rhythms and voluntary ethanol consumption under standard 12:12 light-dark (LD) cycles, and in constant light (LL) and constant darkness (DD). Experiment 1 employed male C3H/He inbred mice, while Experiment 2 employed males and females from a genetically heterogeneous line (WSC). Relative to LD conditions, ethanol intake and ethanol preference were reduced under both LL and DD in both experiments. Because similar effects were seen in both LL and DD, neither circadian disruption nor a classical photoperiodic mechanism are likely to account fully for these findings. Instead, we suggest that the absence of circadian entrainment may function as a mild stressor, resulting in reduced ethanol consumption.
Keywords: ethanol, preference, photoperiod, constant light, constant darkness, circadian, mouse
1. Introduction
Seasonal and geographic variations in photoperiod are associated with alterations in human mood, sleep, appetite, and behavior (Kasper et al., 1989; Wehr, 2001; Yang et al., 2010; Golder and Macy, 2011). Similarly, nocturnal light exposure promotes physiological and behavioral pathologies in humans and in both domesticated and free-living animal populations (Bedrosian and Nelson, 2017; Russart and Nelson, 2018). In laboratory experiments, exposure to various atypical lighting regimens has been shown to induce widespread physiological and behavioral disruption (Stephenson et al., 2012; Bedrosian and Nelson, 2013; Landgraf et al., 2014; LeGates et al., 2014). Several distinct lighting manipulations have been employed in these experiments, including 24-hour light-dark (LD) cycles with unusually long (summer-like) or short (winter-like) photoperiods; LD cycles with non-24 hour periods; constant darkness (DD) and/or constant light (LL); and repeated LD phase shifts mimicking the light exposure patterns associated with jet-lag or shift-work (often referred to as “shift-lag” lighting in the animal literature).
During steady-state entrainment, circadian rhythms maintain stable phase relationships to the environmental LD cycle (“external synchronization”), while at the same time, multiple rhythmic processes within the organism maintain stable (and presumably optimal) phase relationships to one another (“internal synchronization”). Extensive research indicates that loss of external and/or internal synchrony can promote both medical and psychiatric disease (Karatsoeos, 2012; McClung, 2013; Evans and Davidson, 2013; Schnell et al., 2014; Zelinski et al., 2014). It is reasonable, to hypothesize, therefore, that the negative health effects of exposure to atypical lighting regimens may be mediated, at least in part, by circadian disruption. On the other hand, it is highly likely that non-circadian effects of light on sleep, alertness, mood, and neuroendocrine status also contribute to these phenomena (Stephenson et al., 2012; LeGates et al., 2014).
Human alcohol consumption varies as a function of season and latitude (Lemmens and Knibbe, 1993; McGrath and Yahia, 1993; Uitenbroek, 1996; Paschane, 1998; World Health Organization, 2018), and substantial comorbidity is observed between alcohol abuse and seasonal affective disorder (Allen et al., 1993; Sher, 2002; 2004), suggesting that photoperiod may affect human alcohol drinking. In one of the few relevant studies done in mice, Millard and Dole (1983) found reduced voluntary ethanol intake under an LD 6:6 cycle, relative to standard LD 12:12 conditions. Importantly, mice showed high levels of fluid intake only during one of the two daily 6-hour dark periods under LD 6:6, indicating that the circadian clock “interpreted” the LD 6:6 schedule as though it were a 24-hour, long-photoperiod lighting cycle (i.e., LD 18:6), and not as a 12-hour LD cycle. These observations suggest that photoperiod-related effects on ethanol intake may be mediated by differences in circadian entrainment pattern, rather than by differences in total daily light exposure, which was identical under both lighting regimens. This interpretation would be entirely consistent with the more well-studied effects of photoperiod on reproductive and immune functions.
We recently examined voluntary ethanol intake in mice housed under either short (LD 6:18) or long (LD 18:6) photoperiods (Rosenwasser et al., 2015). Consistent with the results of Millard and Dole (1983), mice displayed significantly lower ethanol intake under long relative to short photoperiods. Further, we observed similar effects under both normal and so-called “skeleton” photoperiods, in which the daily light-to-dark and dark-to-light transitions are replaced by brief light pulses in otherwise constant darkness. These results strengthen the argument that photoperiodic modulation of ethanol intake is related to circadian entrainment pattern rather than to the total duration of daily light exposure.
Other studies have examined the effects of constant darkness and/or constant light on voluntary ethanol intake. While early studies reported that ethanol intake is increased in DD relative to standard LD cycles in both rats (Geller, 1971; Burke and Kramer, 1974) and hamsters (Reiter et al., 1974; Geller and Hartmann, 1977), more recent studies have found reduced ethanol intake under DD in both rats (Goodwin et al., 1999) and mice (Trujillo et al., 2011; Rosenwasser and Fixaris, 2013). Similarly inconsistent findings have been seen in LL, including increased, decreased, and unchanged ethanol intake (hamsters: Hammer et al., 2010; rats: Goodwin et al.,1999; mice: Rosenwasser and Fixaris, 2013; Ruby et al., 2014). While it is not clear how to resolve these discrepant findings, it should be noted that both LL and DD are associated with depression- and anxiety-like like behaviors in animal models (Tapia-Osorio et al., 2013; LeGates et al., 2014). Since LL generally results in far greater circadian disruption than DD, and since LL and DD typically have opposite effects on photoperiodic responses, neither circadian disruption nor photoperiodic mechanisms are likely to fully account for the behavioral effects of LL and DD.
The present experiments were designed to further explore the effects of both DD and LL on voluntary ethanol intake in mice, and to extend such observations to include additional genotypes and both sexes. The results of these experiments confirm and extend the generality of previous findings that voluntary ethanol intake is generally lower in both DD and LL, relative to normal LD entrainment conditions.
2. Methods
2.1. Animals and apparatus
Two separate experiments were conducted using similar methods but with different mouse populations. For Experiment 1, male C3H/HeJ inbred mice (N = 10) were obtained at 8 weeks of age from the Jackson Laboratory (Bar Harbor, ME). For Experiment 2, male and female WSC-2 (Withdrawal Seizure Control, replicate 2) mice (N = 15 per sex) were obtained at about 8 weeks of age from the breeding facility of the Portland Alcohol Research Center (Portland, OR). WSC-2 mice were originally derived from an 8-way cross among inbred strains for use as controls for comparison to selectively-bred Withdrawal Seizure Prone (WSP) and Withdrawal Seizure Resistant (WSR) mice (Crabbe et al., 1985), but in this study, WSC mice were employed simply as a non-selected genetically heterogeneous line. This work employed the moderatedrinking C3H/HeJ strain and the moderate-drinking WSC lineage because our previous study (Rosenwasser and Fixaris, 2013) had used the high-drinking C57BL/6J and the low-drinking DBA/2J strains, and because we wanted to maximize the generality of our findings and to confirm that similar effects could be seen across a range of ethanol-preference phenotypes.
Upon arrival in the lab, mice were individually housed in commercial cages equipped with running wheels (Coulbourn Instruments, Whitehall, PA; model ACT 551; wheel diameter = 11.5 cm). Running wheels were freely available throughout the experiments, and wheel-turns were logged in 1-minute bins using the ClockLab interface system (Coulbourn Instruments, Whitehall, PA). The cages were held three per shelf within modified steel supply cabinets equipped with computer-controlled lighting and ventilation fans that also provided masking noise. Each cabinet could hold a total of 15 running-wheel cages, and both males and females were housed within each cabinet. Food (Prolab RMH 3000, PMI Nutrition International, Saint Louis, MO), plain tap water, and 10% (v/v) ethanol solution were all available continuously throughout the experiments.
2.2. Procedures
2.2.1. Experiment 1
Mice in Experiment 1 were maintained under a series of lighting conditions for six weeks per condition, as follows: LD 12:12 (LD1); DD; LD 12:12 (LD2); LL; LD 12:12 (LD3). Water and ethanol intake and body weight was recorded on a weekly basis, and bottle positions were switched after each measurement. As is typical in studies of 2-bottle preference drinking, ethanol intake is expressed as g/kg/day, while water intake is reported as ml/day. In addition, ethanol, preference ratios were computed by dividing the intake of ethanol solution in ml by total fluid intake. Circadian period and amplitude were estimated for each animal in each lighting condition using the Lomb-Scargle periodogram, as implemented in the ClockLab program.
2.2.2. Experiment 2
Mice in Experiment 2 were maintained under one of two distinct sequences of lighting conditions for four weeks per condition, as follows: Sequence 1: LD 12:12 (LD1); DD; LD 12:12 (LD2); Sequence 2: LD 12:12 (LD1); LL; LD 12:12 (LD2). All other procedures were identical to those employed in Experiment 1.
2.2.3. Statistics
Experiment 1: Data were analyzed using repeated-measures ANOVA, with lighting condition as the within-subjects factor. Following identification of significant effects of lighting condition, specific pairs of conditions were compared using paired t-tests with alpha set at 0.05, adjusted for multiple comparisons. In Experiment 2, the two sequences were analyzed separately using mixed ANOVA, with lighting condition as a within-subjects factor and sex as a between-groups factor. Identification of sex effects was followed by separate tests for simple main effects in each sex using ANOVA, and significant effects of lighting conditions were followed by alpha-adjusted pairwise t-tests, as in Experiment 1.
3. Results
3.1. Experiment 1
3.1.1. Circadian activity rhythms
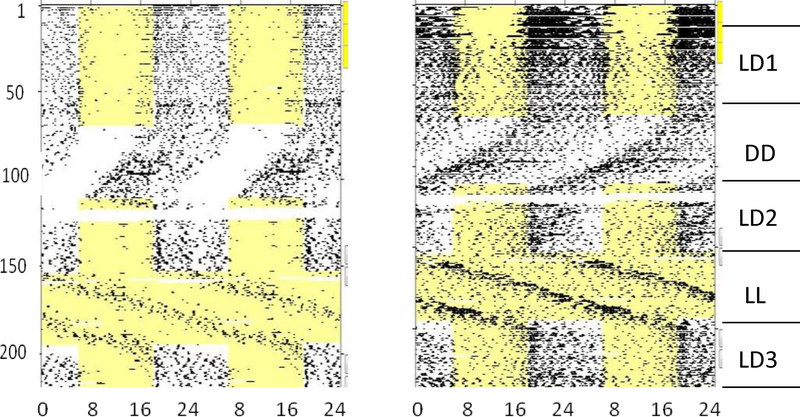
Qualitative and quantitative aspects of circadian activity rhythms conformed to expectations for nocturnal mice. Thus, male C3H mice displayed largely nocturnal activity patterns under LD cycles and free-running circadian rhythms under both DD and LL (Fig. 1). As is typical for mice, free-running periods were generally shorter than 24 hours in DD and longer than 24 hours in LL. While it is well known that LL of sufficient intensity can induce dramatic circadian disruption (Rosenwasser and Adler, 1986), animals in the present experiment generally maintained clearly distinct activity and rest phases in both DD and LL.
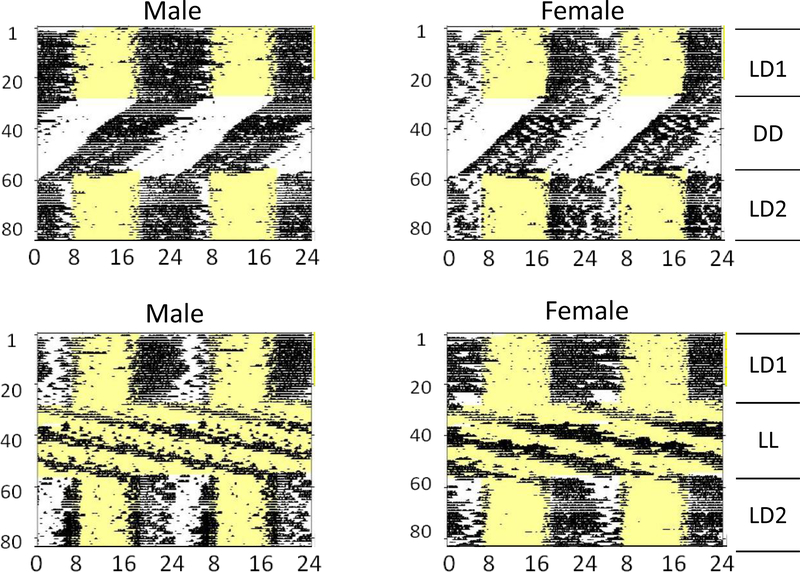
Figure 1.
Raster-style circadian actograms for two representative male C3H/He mice, illustrating the temporal patterning of wheel-running activity over the entire course of Experiment 1. Time of day is double-plotted (48-hour span) along the x-axis, and successive experimental days are plotted top to bottom along the y-axis. A symbol is plotted for each 10-minute epoch, with height proportional to the number of wheel-turns in that time bin; thus, dark areas indicate times of high activity. Lighting conditions are indicated to the right. LD1, LD2 and LD3: periods during which mice were held under light-dark 12:12; DD: constant darkness; LL: constant light.
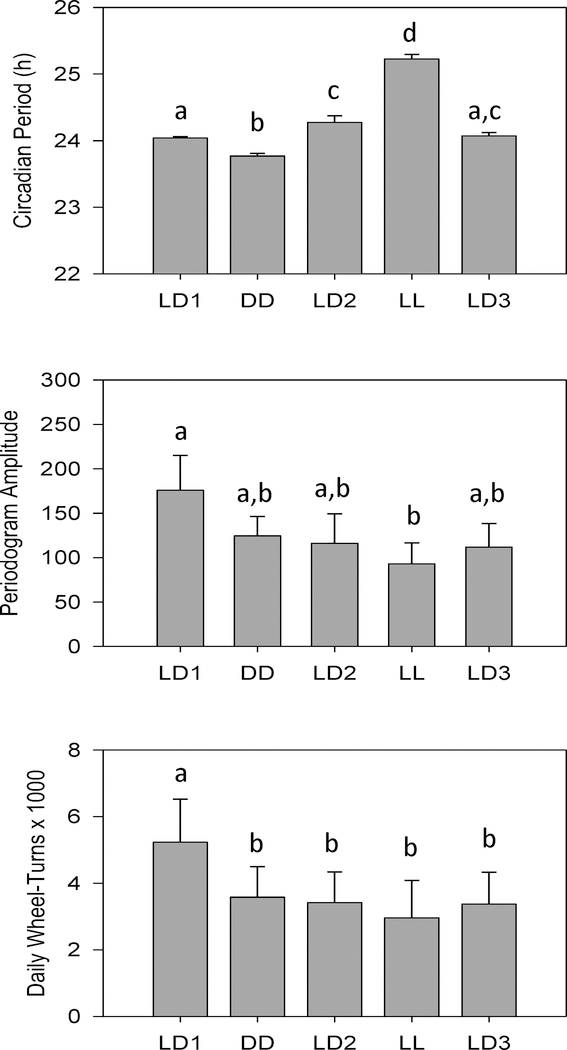
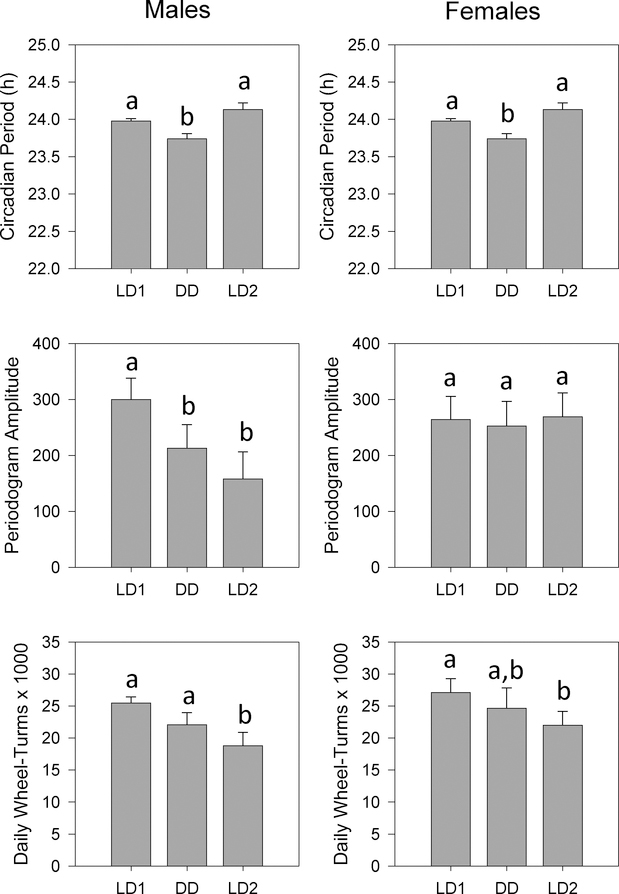
Quantitative analysis of circadian activity rhythms confirmed the qualitative observations described above (Fig. 2). Circadian period varied across lighting conditions (F4,36 = 89.96, p < 0.001) and was significantly shorter in DD and significantly longer in LL than in any of the three LD conditions. Unexpectedly, period was longer in LD2 than in LD1, possibly reflecting incomplete resynchronization in some animals. Periodogram amplitude (F4,36 = 4.394, p = 0.005) and daily wheel-turns (F4,36 = 6.135, p = 0.001) also varied across lighting conditions, in that both tended to decrease over the course of the experiment and to be lowest in LL.
Figure 2.
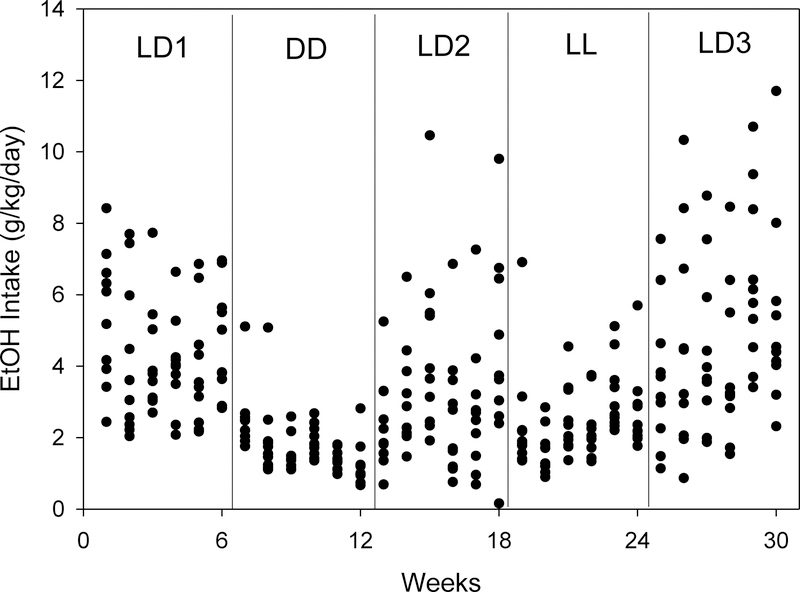
Ethanol intake (g/kg/day) for each individual male C3H mouse (n=10) across the 30 weeks of the experiment. Animals were maintained under a series of five successive lighting conditions for six weeks per condition. LD1, LD2, and LD3 refer to maintenance in light-dark 12:12 conditions; DD, maintenance in continuous darkness; LL, maintenance in continuous light.
3.1.2. Fluid consumption
Figure 3 shows ethanol intake for each individual animal over the 30-week course of the experiment. While ethanol intake varied among individuals, especially under LD conditions, changes in ethanol intake across lighting conditions generally emerged within the first week of each 6-week condition. Thus, ethanol intake was consistently lower in the first week of both LL and DD relative to the preceding LD condition, and was consistently higher in the first week of LD2 and LD3 relative to the immediately preceding non-entrained condition. Further, there were no apparent trends in intake across weeks within conditions. Thus, data were collapsed by averaging across weeks within each of the five sequential lighting conditions for subsequent statistical analyses.
Figure 3.
Means +/− standard errors for circadian activity parameters. Circadian Period: top panel; Periodogram Amplitude: middle panel; Daily Wheel-Turns: bottom panel. Data are collapsed across weeks within each lighting condition. Lighting conditions are identified as in Fig. 2. Superscripted letters are used to indicate significant pairwise differences between conditions; bars with no shared letters are significantly different from one another.
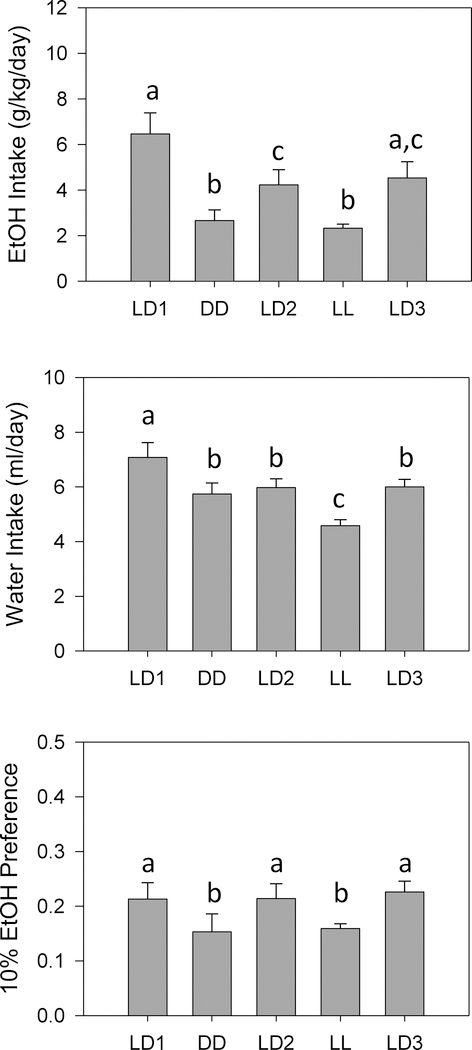
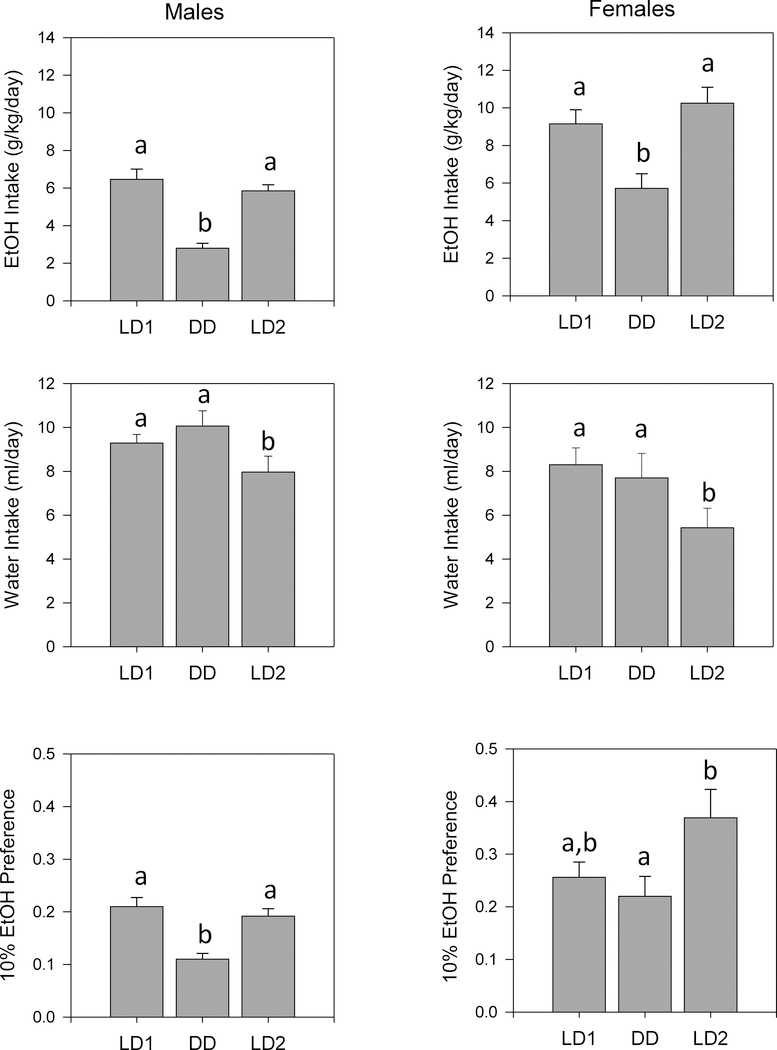
ANOVA revealed significant effects of lighting condition on both ethanol intake (F4,36 = 12.284, p < 0.001) and ethanol preference (F4,36 = 2.689, p = 0.046) (Figure 4). Pairwise comparisons showed that ethanol intake and ethanol preference were significantly lower in both DD and LL than in any of the three LD conditions, while no differences were seen between DD and LL. ANOVA also revealed a significant effect of lighting condition on water intake (F4,36 = 13.977, p < 0.001) (Figure 4), while pairwise comparisons showed that water intake was higher in LD1 and lower in LL than in any other condition.
Figure 4.
Means +/− standard errors for fluid intake parameters. Ethanol Intake: top panel; Water Intake: middle panel, 10% Ethanol Preference ratio: bottom panel. All other conventions as in Fig. 2.
3.2. Experiment 2
3.2.1. Circadian activity rhythms
Both male and female WSC mice displayed activity patterns that were typical for nocturnal mice, with free-running periods generally less than 24 hours in DD (Sequence 1) and greater than 24 hours in LL (Sequence 2) (Fig. 5). WSC activity rhythms were characterized by clearly distinct activity and rest phases and by very high levels of activity. As in Experiment 1, all animals displayed coherent rhythmicity in both DD and in LL, and there were no obvious differences between males and females.
Figure 5.
Raster-style circadian actograms for two representative WSC male mice (left) and two representative female WSC mice (right) from Experiment 2. All other conventions as in Figure 1.
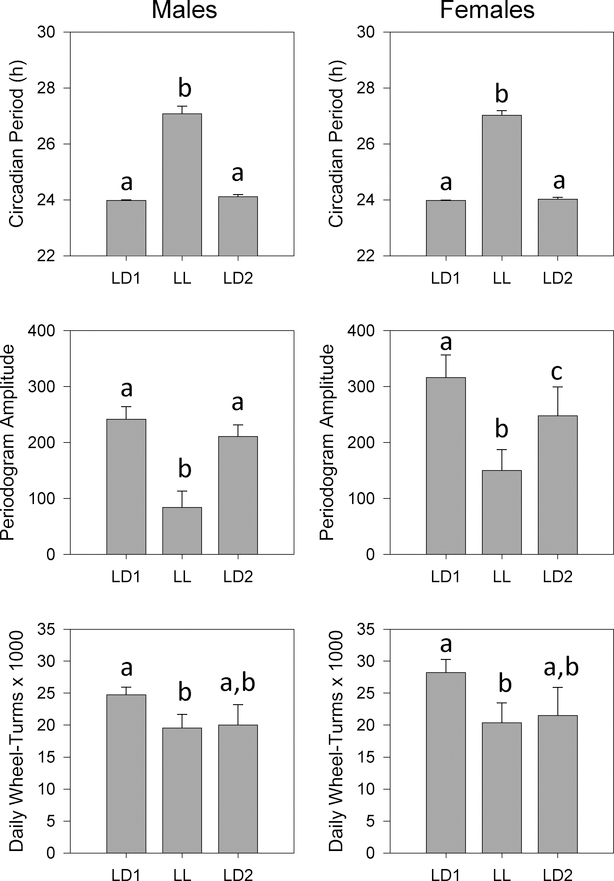
ANOVA revealed significant effects of lighting condition on circadian period for both Sequence 1 (F2,24 = 17.477, p < 0.001) and Sequence 2 (F2,26 = 409.455, p < 0.001), but no main effects or interactions with sex (Figs. 6, 7). Pairwise tests showed that periods were shorter in DD (Sequence 1) and longer in LL (Sequence 2) than in either LD1 or LD2.
Figure 6.
Means +/− standard errors for circadian activity parameters in male and female WSC mice (n=15 per sex) tested in Sequence 1 (LD1, DD, LD2). Circadian Period: top panels; Periodogram Amplitude, middle panels; Daily Wheel-Turns: bottom panels. Males are on the left and females on the right. Other conventions as in Fig. 2.
Figure 7.
Means +/− standard errors for circadian activity parameters in male and female WSC mice (n=15 per sex) tested in Sequence 2 (LD1, LL, LD2). Circadian Period: top panels; Periodogram Amplitude, middle panels; Daily Wheel-Turns: bottom panels. Males are on the left and females on the right. Other conventions as in Fig. 2.
Analysis of periodogram amplitude revealed significant interactions between lighting condition and sex for both Sequence 1 (F2,24 = 3.484, p = 0.047) and Sequence 2 (F2,26 = 17.191, p < 0.001) (Figs. 6, 7). In Sequence 1, males showed a progressive decrease in amplitude across the experiment (F2,10 = 6.457, p = 0.016), while amplitudes were stably maintained in females (Fig. 6). In contrast, amplitudes were significantly reduced under LL relative to both LD1 and LD2 in both males (F2,14 = 19.463, p < 0.001) and females (F2,12 = 18.293, p < 0.001) (Fig. 7). Thus, while DD appear to not affect periodogram amplitude, amplitude was significantly reduced in LL relative to LD in both males and females.
Analysis of daily activity levels revealed significant main effects of lighting condition for both sequences (Sequence 1: F2,22 = 37.071, p < 0.001; Sequence 2: F2,22 = 12.123, p < 0.001) (Figs. 6, 7). While activity levels tended to decrease across the course of the experiment in both Sequences, examination of pairwise comparisons indicated that activity levels were reduced under LL (Fig. 7) but not under DD (Fig. 6), relative to the preceding LD1 condition. Finally, females ran marginally more than males in Sequence 1 (F1,11 = 4.836, p = 0.050) but not in Sequence 2.
3.2.2. Fluid consumption
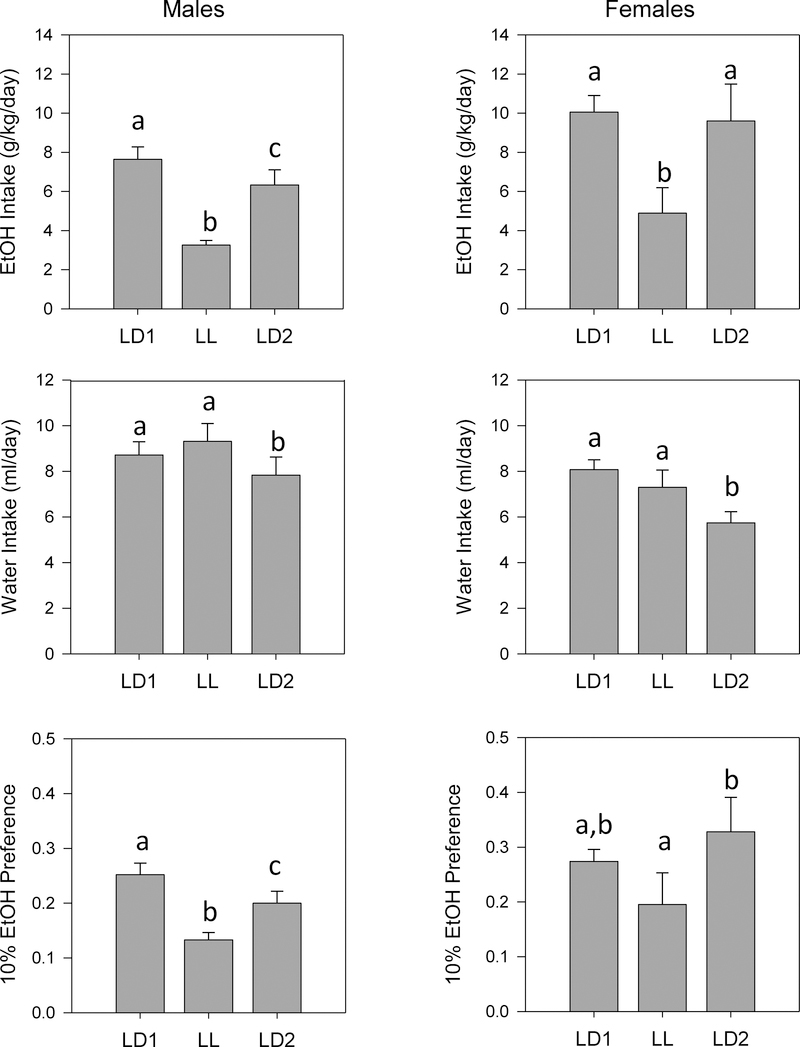
Lighting condition by sex ANOVA revealed significant effects of lighting condition on ethanol intake for both Sequence 1 (F2,24 = 49.880, p < 0.001) and Sequence 2 (F2,26 = 50.012, p < 0.001), a significant effect of sex for Sequence 1 (F1,12 = 14.940, p = 0.002), and a non-significant trend in the same direction for Sequence 2 (p = 0.091); however, no lighting by sex interactions were found (Figs. 8, 9). Ethanol intake was reduced in both DD (Fig. 8) and LL (Fig. 9) relative to both LD1 and LD2, in both males and females.
Figure 8.
Means +/− standard errors for fluid intake parameters in male and female WSC mice (n=15 per sex) tested in Sequence 1 (LD1, DD, LD2). Ethanol Intake: top panels; water intake: middle panels; 10% Ethanol Preference ratio: bottom panels. Males are on the left and females on the right. Other conventions as in Fig. 2.
Figure 9.
Means +/− standard errors for fluid intake parameters in male and female WSC mice (n=15 per sex) tested in Sequence 2 (LD1, LL, LD2). Ethanol Intake: top panels; water intake: middle panels; 10% Ethanol Preference ratio: bottom panels. Males are on the left and females on the right. Other conventions as in Fig. 2.
The effects of lighting condition on ethanol preference was very similar to those seen for ethanol intake. Thus, ANOVA revealed significant effects of lighting condition on ethanol preference for both Sequence 1 (F2,24 = 13.599, p < 0.001) and Sequence 2 (F2,26 = 21.799, p < 0.001), and a significant effect of sex for Sequence 1 only (F1,12 = 6.639, p = 0.024) (Figs. 8, 9). In contrast to the effects on ethanol intake, however, significant lighting condition by sex interactions were seen for both Sequence 1 (F2,24 = 4.551, p = 0.021) and Sequence 2 (F2,26 = 4.594, p = .020). Examination of pairwise comparisons indicated that ethanol preference was significantly reduced in both DD and LL relative to both LD1 and LD2 in males, while in females, ethanol preference was significantly reduced in both DD and LL only when compared to LD2 (Figs. 8, 9).
ANOVA revealed significant effects of lighting condition on water intake in both Sequence 1 (F2,24 = 26.713, p < 0.001) and Sequence 2 (F2,26 = 26.995, p < 0.001) (Figs. 8, 9). While there were no main effects of sex in either sequence, there was a significant lighting condition by sex interaction for Sequence 2 (F2,26 = 6.063, p = 0.007) and a non-significant (p = 0.066) trend in the same direction in Sequence 1. Analysis of pairwise comparisons showed that water intake gradually decreased across lighting conditions in females in, and was lower in LD2 than in any other condition in males, in both Sequence 1 and Sequence 2.
4. Discussion
The present results confirm previous reports that voluntary ethanol consumption is reduced in both DD and LL, relative to standard LD conditions, in both rats (Goodwin et al., 1999) and mice (Rosenwasser and Fixaris, 2013). Taken together with the results of Rosenwasser and Fixaris (2013), we have now seen reduced ethanol consumption under DD in high- (C57BL/6J), low- (DBA/2J) and moderate-drinking (C3H/HeJ, WSC) genotypes, and reduced ethanol consumption under LL in all but the DBA/2J mice. Further, in the case of the WSC mice, males and females showed essentially identical responses to both DD and LL. Thus, we have identified a novel, reliable and generalizable environmental manipulation that significantly alters free-choice ethanol drinking in mice, regardless of strain and sex differences in innate ethanol preference.
Nevertheless, our results appear to contrast with early studies reporting increased ethanol intake under DD in rats (e.g., Geller, 1971, Burke and Kramer, 1974). On the other hand, the Geller (1971) study has been criticized as being poorly controlled for possible spontaneous changes with time (Sinclair, 1972; Choca et al., 1977). While a number of early studies also reported increased ethanol intake in Syrian hamsters under DD (Reiter et al.,1974; Geller and Hartmann, 1977), this species – unlike mice and rats – is known to display strongly photoperiodic reproductive and immune responses. Given the reported failure of hamsters to alter their ethanol intake in LL relative to LD (Hammer et al., 2010; Ruby et al., 2014), it would not be surprising to find species differences in the response to DD as well.
Animals in the present study were maintained in running-wheel cages throughout the experiments. While this allowed us to characterize entrained and free-running circadian activity rhythms under the various lighting regimens, access to running wheels has itself been reported to alter free-choice ethanol intake (McMillan et al., 1995; Ehringer et al., 2009; Hammer et al., 2010; Piza-Palma et al., 2014). In two previous studies, however, we found that wheel-housed mice displayed increased water intake, but no significant effect on ethanol intake (Rosenwasser et al., 2012; 2015). Similarly, Ehringer et al. (2009) found that reduced ethanol preference in wheel-housed male mice was mainly due to increased water intake, rather than to changes in ethanol consumption per se. Thus, while it is possible that the presence of running wheels influenced the present results, it seems unlikely that running-wheel access is a major factor limiting the generalizability of these findings.
Another factor potentially limiting the generalizability of our findings in our use of the 2-bottle continuous-access free-choice model of voluntary ethanol intake. While this model has been widely employed to study the genetic, pharmacological, and environmental factors influencing voluntary ethanol intake, it must be noted that 2-bottle free-choice drinking is thought to model non-pathological, non-dependent, moderate drinking in humans (Tabakoff and Hoffman, 2000; Sanchis-Segura and Spanagel, 2006; Green and Grahame, 2008). With a few possible exceptions in selectively-bred lines, the literature indicates that rats and mice do not become either intoxicated or ethanol-dependent under these conditions, and researchers have developed numerous other models to better simulate factors such as dependence, tolerance, and withdrawal in experimental animals (Tabakoff and Hoffman, 2000; Sanchis-Segura and Spanagel, 2006). While two-bottle free-choice drinking is known to be influenced by factors including the taste and caloric value of the ethanol stimulus, free-choice intake is strongly correlated with tests of ethanol reward and aversion (Green and Grahame, 2008). Therefore, while mice in the present study showed educed ethanol intake under LL and DD, it is certainly possible that different results would be seen with models of excessive or dependence-based drinking.
A growing body of research has revealed that atypical laboratory lighting regimens – including both DD and LL – are associated with affective disturbances in a variety of animal models (Stephenson et al., 2012; Bedrosian and Nelson, 2013; Landgraf et al., 2014; LeGates et al., 2014). Since ethanol intake is known to be strongly modulated by affective state, we believe that our findings may be directly related to other work on the affective consequences of lighting manipulations. Several non-exclusive chronobiological mechanisms are commonly advanced to explain the effects of atypical lighting schedules on affective behavior. These include circadian disruption, alterations in nocturnal melatonin secretion, and photoperiodism. However, since LL and DD have dissimilar effects on circadian rhythms, melatonin secretion, and photoperiodic responses, it is difficult to see how these factors could account for the apparently similar affective-behavioral effects of both LL and DD. In the present study, we concurrently assessed circadian activity rhythms and ethanol intake in animals exposed to different lighting conditions, and found that changes in circadian parameters did not covary with changes in ethanol intake. While ethanol intake was reduced in both LL and DD, free-running circadian period lengthened in LL and shortened in DD, while periodogram amplitude and locomotor activity were generally reduced in LL but not in DD. Thus, while chronobiological factors may contribute to the effects seen in the present study, it seems unlikely that any specific chronobiological hypothesis can account fully for the alterations in ethanol intake seen in both DD and LL.
While it might seem counterintuitive that lighting conditions that have been associated with increased depression- and anxiety-like behaviors would also be associated with reduced, rather than increased ethanol intake, the relationship between stress and ethanol intake is by no means simple. Thus, an extensive review of the literature (Becker et al., 2011) revealed that environmental stressors are at least as likely to reduce as to increase voluntary ethanol intake, especially in studies (such as this one) examining free-choice drinking in non-dependent animals. Indeed, a reasonable hypothesis is that stress-related affective disruption may reduce non-dependent, reward-based ethanol intake, while at the same time promoting ethanol intake in models of ethanol dependence (Becker et al., 2011). These considerations suggest the hypothesis that the reduced ethanol intake seen under LL and DD in the present study and in our previous work (Rosenwasser and Fixaris, 2013), as well as the reduced ethanol intake in rats housed under chronic shift-lag lighting regimens (Clark et al., 2007; Rosenwasser et al., 2010), are due to dysphoric effects induced by the stressfulness of living in the absence of a stable environmental LD cycle. Such a hypothesis could also account for recent findings that health and longevity are compromised in animals that display free-running periods deviating substantially from 24 hours in either direction (Wyse et al., 2010; Libert et al., 2012). Thus, both physiological and psychological homeostasis may be optimized when the circadian clock is entrained to a strict 24-hour period.
Taken together with previous studies, the present results confirm that maintenance under atypical lighting regimens, including DD and LL, extreme long and short photoperiods, and shift-lag lighting, can alter voluntary ethanol intake. These effects may be related to changes in affective behavior seen in response to similar lighting manipulations, but the specific roles of chronobiological and non-chronobiological factors remains to be elucidated. Finally, these results may be relevant to understanding the seasonal and geographic variations in alcohol consumption in diverse human populations.
Voluntary ethanol intake is reduced in mice housed under either constant darkness (DD) or constant light (LL), relative to animals maintained in a standard light-dark (LD) cycle.
These results are difficult to explain based on circadian disruption, photoperiodism, or direct effects of light and darkness on arousal.
Instead, we suggest that the lack of circadian entrainment per se may be mildly stressful.
Alternately, the suppressive effects of DD and LL may be mediated by separate and distinct mechanisms.
Acknowledgements
Supported in part by NIH R24AA020245 to John C. Crabbe.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Allen JM, Lam RW, Remick RA, Sadovnick AD Depressive symptoms and family history in seasonal and nonseasonal mood disorders. Am J Psychiatry. 1993,150:443–8. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl). 2011,218:131–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian TA, Nelson RJ Influence of the modern light environment on mood. Mol Psychiatry. 2013,18:751–7. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Nelson RJ Timing of light exposure affects mood and brain circuits. Transl Psychiatry. 2017,7:e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke LP, Kramer SZ Effects of photoperiod, melatonin and pinealectomy on ethanol consumption in rats. Pharmacol Biochem Behav. 1974,2:459–63. [DOI] [PubMed] [Google Scholar]
- Choca JP, Wilson AS, Garcia TJ Effects of shock and darkness on alcohol consumption by rats. J Stud Alcohol. 1977,38:2184–7. [DOI] [PubMed] [Google Scholar]
- Clark JW, Fixaris MC, Belanger GV, Rosenwasser AM Repeated light-dark phase shifts modulate voluntary ethanol intake in male and female high alcohol-drinking (HAD1) rats. Alcohol Clin Exp Res. 2007,31:1699–706. [DOI] [PubMed] [Google Scholar]
- Ehringer MA, Hoft NR, Zunhammer M Reduced alcohol consumption in mice with access to a running wheel. Alcohol. 2009,43:443–52. [DOI] [PubMed] [Google Scholar]
- Evans JA, Davidson AJ Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Transl Sci. 2013,119:283–323. [DOI] [PubMed] [Google Scholar]
- Geller I Ethanol preference in the rat as a function of photoperiod. Science. 1971,173:456–9. [DOI] [PubMed] [Google Scholar]
- Geller I, Hartmann RJ Alteration of ethanol preference in hamsters: effects of photoperiod and 5-hydroxytryptophan. Adv Exp Med Biol. 1977,85B:223–33. [DOI] [PubMed] [Google Scholar]
- Golder SA, Macy MW Diurnal and seasonal mood vary with work, sleep, and daylength across diverse cultures. Science. 2011,333:1878–81. [DOI] [PubMed] [Google Scholar]
- Goodwin FL, Amir S, Amit Z Environmental lighting has a selective influence on ethanol intake in rats. Physiol Behav. 1999,66:323–8. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2011,42:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer SB, Ruby CL, Brager AJ, Prosser RA, Glass JD Environmental modulation of alcohol intake in hamsters: effects of wheel-running and constant light exposure. Alcohol Clin Exp Res. 2010,34:1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN Effects of circadian disruption on mental and physical health. Curr Neurol Neurosci Rep. 2012,12:218–25. [DOI] [PubMed] [Google Scholar]
- Kasper S, Wehr TA, Bartko JJ, Gaist PA, Rosenthal NE Epidemiological findings of seasonal changes in mood and behavior. A telephone survey of Montgomery County, Maryland. Arch Gen Psychiatry. 1989,46:823–33. [DOI] [PubMed] [Google Scholar]
- Landgraf D, McCarthy MJ, Welsh DK The role of the circadian clock in animal models of mood disorders. Behav Neurosci. 2014,128:344–59. [DOI] [PubMed] [Google Scholar]
- LeGates TA, Fernandez DC, Hattar S Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014,15:443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmens PH, Knibbe RA Seasonal variation in survey and sales estimates of alcohol consumption. J Stud Alcohol. 1993,54:157–63. [DOI] [PubMed] [Google Scholar]
- Libert S, Bonkowski MS, Pointer K, Pletcher SD, Guarente L Deviation of innate circadian period from 24 h reduces longevity in mice. Aging Cell. 2012,11:794800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA How might circadian rhythms control mood? Let me count the ways. Biol Psychiatry. 2013,74:242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath RE, Yahia M Preliminary data on seasonally related alcohol dependence. J Clin Psychiatry. 1993,54:260–2. [PubMed] [Google Scholar]
- McMillan DE, McClure GY, Hardwick WC Effects of access to a running wheel on food, water and ethanol intake in rats bred to accept ethanol. Drug Alcohol Depend. 1995,40:1–7. [DOI] [PubMed] [Google Scholar]
- Millard WJ, Dole VP Intake of water and ethanol by C57BL mice: effect of an altered light-dark schedule. Pharmacol Biochem Behav. 1983,18:281–4. [DOI] [PubMed] [Google Scholar]
- Paschane D Variability of substance abuse. Global variability of substance abuse: is latitude a unique etiological factor? Int J Circumpolar Health. 1998,57:228–38. [PubMed] [Google Scholar]
- Piza-Palma C, Barfield ET, Brown JA, Hubka JC, Lusk C, Schonhar CA, et al. Oral self-administration of EtOH: sex-dependent modulation by running wheel access in C57BL/6J mice. Alcohol Clin Exp Res. 2014,38:2387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Blum K, Wallace JE, Merritt JH Pineal gland: evidence for an influence on ethanol preference in male Syrian hamsters. Comp Biochem Physiol A Comp Physiol. 1974,47:11–6. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Adler NT Structure and function in circadian timing systems: evidence for multiple coupled circadian oscillators. Neurosci Biobehav Rev. 1986,10:431–48. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Clark JW, Fixaris MC, Belanger GV, Foster JA Effects of repeated light-dark phase shifts on voluntary ethanol and water intake in male and female Fischer and Lewis rats. Alcohol. 2010,44:229–37. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Fixaris MC, Crabbe JC, Brooks PC, Ascheid S Escalation of intake under intermittent ethanol access in diverse mouse genotypes. Addiction Biol. 2012,18:496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM, Fixaris MC Chronobiology of alcohol: studies in C57BL/6J and DBA/2J inbred mice. Physiol Behav. 2013,110-111:140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM, Fixaris MC, McCulley WD 3rd. Photoperiodic modulation of voluntary ethanol intake in C57BL/6 mice. Physiol Behav. 2015,147:342–7. [DOI] [PubMed] [Google Scholar]
- Ruby CL, Vadnie CA, Hinton DJ, Abulseoud OA, Walker DL, O’Connor KM, et al. Adenosinergic regulation of striatal clock gene expression and ethanol intake during constant light. Neuropsychopharmacology. 2014,39:2432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russart KLG, Nelson RJ Light at night as an environmental endocrine disruptor. Physiol. Behav 2018,190:82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006,11:2–38. [DOI] [PubMed] [Google Scholar]
- Schnell A, Albrecht U, Sandrelli F Rhythm and mood: relationships between the circadian clock and mood-related behavior. Behav Neurosci. 2014,128:326–43. [DOI] [PubMed] [Google Scholar]
- Sher L Relationships between seasonality and alcohol use: a genetic hypothesis. Med Hypotheses. 2002,59:85–8. [DOI] [PubMed] [Google Scholar]
- Sher L Alcoholism and seasonal affective disorder. Compr Psychiatry. 2004,45:51–6. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Geller I Ethanol consumption by rats under different lighting conditions. Science. 1972,175:1143–4. [DOI] [PubMed] [Google Scholar]
- Stephenson KM, Schroder CM, Bertschy G, Bourgin P Complex interaction of circadian and non-circadian effects of light on mood: shedding new light on an old story. Sleep Med Rev. 2012,16:445–54. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL Animal models in alcohol research. Alcohol Res Health. 2000,24:77–84. [PMC free article] [PubMed] [Google Scholar]
- Tapia-Osorio A, Salgado-Delgado R, Angeles-Castellanos M, Escobar C Disruption of circadian rhythms due to chronic constant light leads to depressive and anxiety-like behaviors in the rat. Behav Brain Res. 2013,252:1–9. [DOI] [PubMed] [Google Scholar]
- Trujillo JL, Do DT, Grahame NJ, Roberts AJ, Gorman MR Ethanol consumption in mice: relationships with circadian period and entrainment. Alcohol 2011,45:147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitenbroek DG Seasonal variation in alcohol use. J Stud Alcohol. 1996,57:47–52. [DOI] [PubMed] [Google Scholar]
- Wehr TA Photoperiodism in humans and other primates: evidence and implications. J Biol Rhythms. 2001,16:348–64. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global status report on alcohol and health 2018. WHO 2018, Geneva, Switzerland, https://www.who.int/substance_abuse/publications/global_alcohol_report/en/ [Google Scholar]
- Wyse CA, Coogan AN, Selman C, Hazlerigg DG, Speakman JR Association between mammalian lifespan and circadian free-running period: the circadian resonance hypothesis revisited. Biol Lett. 2010,6:696–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang AC, Huang NE, Peng CK, Tsai SJ Do seasons have an influence on the incidence of depression? The use of an internet search engine query data as a proxy of human affect. PLoS One. 2010,5:e13728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski EL, Deibel SH, McDonald RJ The trouble with circadian clock dysfunction: multiple deleterious effects on the brain and body. Neurosci Biobehav Rev. 2014,40:80–101. [DOI] [PubMed] [Google Scholar]