Figure 4:
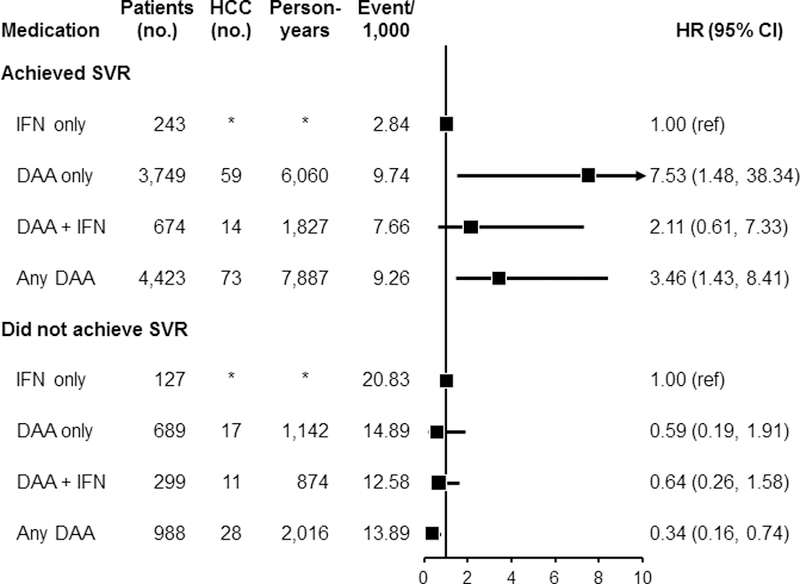
Association between use of antiviral medication for HCV and HCC risk separately among those who achieved sustained virological response (SVR) and those who did not achieve SVR. Analyses were performed after inverse probability of treatment weighting (IPTW) of treatment-related factors. Among patients who achieved SVR: after the IPTW, additional adjustments were performed for age, time from treatment to HCC development, Charlson comorbidity index, race, ribavirin, cirrhosis type, substance use, insurance type, and BMI. For the comparison between DAA + IFN vs. IFN-only, additional adjustment was performed for time from treatment to HCC development after the IPTW. For the comparison between any DAA and IFN-only, additional adjustments were performed for age, time from treatment to HCC development, Charlson comorbidity index, gender, census region, ribavirin, cirrhosis type, insurance type, and smoking status after IPTW. Among patients who did not achieve SVR, the same factors were adjusted for each comparison as was done for those who achieved SVR.
Abbreviations: DAA, direct acting antiviral medication; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IFN, interferon; SVR, sustained virological response
