Abstract
Anti-Müllerian hormone (Amh, or Müllerian-inhibiting substance, Mis), a member of TGF-β superfamily, has been well documented in some vertebrates as initiator or key regulator in sexual development, and particularly in fish. However, its functional role has not yet been identified in reptiles. Here, we characterized the Amh gene in the Chinese soft-shelled turtle Pelodiscus sinensis, a typical reptilian species exhibiting ZZ/ZW sex chromosomes. The messenger RNA of Amh was initially expressed in male embryonic gonads by stage 15, preceding gonadal sex differentiation, and exhibited a male-specific expression pattern throughout embryogenesis. Moreover, Amh was rapidly upregulated during female-to-male sex reversal induced by aromatase inhibitor letrozole. Most importantly, Amh loss of function by RNA interference led to complete feminization of genetic male (ZZ) gonads, suppression of the testicular marker Sox9, and upregulation of the ovarian regulator Cyp19a1. Conversely, overexpression of Amh in ZW embryos resulted in female-to-male sex reversal, characterized by the formation of a testis structure, ectopic activation of Sox9, and a remarkable decline in Cyp19a1. Collectively, these findings provide the first solid evidence that Amh is both necessary and sufficient to drive testicular development in a reptilian species, P. sinensis, highlighting the significance of the TGF-β pathway in reptilian sex determination.
Keywords: Anti-Müllerian hormone, testicular differentiation, sex determination, sex reversal, Genetics of Sex, Pelodiscus sinensis
IN vertebrates, sex determination and gonadal differentiation generally follows the orderly expression of a series of sex-specific genes, which is triggered by the primary sex-determining signal. Since the initial discovery of Sry in eutherian mammal (Koopman et al. 1990; Sinclair et al. 1990; Koopman et al. 1991), several sex-determining genes have been identified in some vertebrate species, such as Dmrt1 in chicken (Smith et al. 2009; Lambeth et al. 2014); Dmw in frog (Yoshimoto et al. 2008); Foxl2 in goat (Boulanger et al. 2014); and Dmy (Matsuda et al. 2002; Nanda et al. 2002), Amhr2 (Kamiya et al. 2012), SdY (Yano et al. 2012), Gsdf (Myosho et al. 2012), Sox3 (Takehana et al. 2014), Gdf6Y (Reichwald et al. 2015), Amhy (Hattori et al. 2012; Li et al. 2015), and Dmrt1 (Chen et al. 2014) in fish. Among these genes, Amhy, Amhr2, and Gsdf are from the transforming growth factor β (TGF-β) signaling pathway, suggesting a conserved role of this pathway in primary sex determination in fish. However, whether the TGF-β pathway plays a critical role in reptilian sex determination and differentiation has not yet been reported.
Anti-Müllerian hormone (Amh), also known as Müllerian-inhibiting substance (Mis), is a hormone-related gene belonging to the TGF-β superfamily. The Amh gene has been found and cloned in various vertebrates of different evolutionary positions, such as mouse (King et al. 1991), chicken (Neeper et al. 1996), American alligator (Western et al. 1999), medaka (Klüver et al. 2007), and tilapia (Shirak et al. 2006). It functions by binding with the type II receptor (AmhrII), which in turn induces the formation of receptor polymers to activate downstream target genes (Josso et al. 2001; Rey et al. 2003; Johnson et al. 2008). In mammals, the Amh gene is expressed in Sertoli cells of embryonic testes, and is responsible for the regression of the Müllerian ducts, but it is not detected during female embryonic development (Josso et al. 2001). In chicken, expression level of Amh is consistently higher in males than in females over the period of gonadal sex differentiation (Smith et al. 1999); however, knockdown of Amh in chicken ZZ embryos does not alter gonadal development (Lambeth et al. 2015). Despite the lack of Müllerian ducts in most teleost fish, the sexually dimorphic expression pattern of Amh and AmhrII is also detected in developing or mature gonads (Miura et al. 2002; Yoshinaga et al. 2004; Wu et al. 2010; Eshel et al. 2014). Deletion of Amhy in Patagonian pejerrey and Amhr2 in Takifugu rubripes, both residing on the Y sex chromosome, results in male-to-female sex reversal, thus rendering these two genes as male sex-determining genes (Hattori et al. 2012; Kamiya et al. 2012). Correlative studies in reptiles show that Amh exhibits male-specific embryonic expression, preceding the gonadal sex differentiation, in the red-eared slider turtle (Shoemaker et al. 2007), painted turtle (Radhakrishnan et al. 2017), and American alligator (Western et al. 1999). These observations suggest a possible upstream position of Amh in the male pathway of reptiles, and its functional role in determining the gonadal sexual fate needs to be elucidated.
Chinese soft-shelled turtle Pelodiscus sinensis exhibiting ZZ/ZW genetic sex-determining system has been recently emerged as an ideal turtle model for investigating reptilian sex determination and differentiation, because of the well-established genetic modulation technique (Ge et al. 2017; Sun et al. 2017) and available genome resource (Wang et al. 2013). In this study, we found that knockdown of Amh by RNA interference resulted in male-to-female sex reversal in P. sinensis. Conversely, overexpression of Amh led to complete masculinization of female genetic turtles, indicating a both necessary and sufficient role of Amh to drive testicular development in a reptilian species.
Materials and Methods
Egg incubation and tissue collection
Freshly laid Chinese soft-shelled turtle (P. sinensis) eggs were obtained from the Dafan turtle farm (Zhejiang, China). Fertilized eggs were placed in egg incubators at 31°, with humidity maintained at 75–85%. During the incubation process, embryos of different developmental stages [stage 14 (14 days), stage 15 (16 days), stage 16 (18 days), stage 17 (20 days), stage 18 (22 days), stage 19 (24 days), stage 20 (26 days), stage 21 (28 days), stage 25 (40 days), stage 27 (48 days; hatching)], which were identified according to criteria established by Tokita and Kuratani (2001), were removed from eggshells, decapitated, and placed in PBS for gonad-mesonephros complexes (GMCs) and whole-gonad collection. GMCs were fixed in 4% paraformaldehyde overnight at 4°, dehydrated through 50% ethanol, and then stored in 70% ethanol at 4° until paraffin embedding and sectioning was performed. Gonads were broken up thoroughly and immersed in TRIzol reagent (Invitrogen, Carlsbad, CA) for total RNA isolation. Meanwhile, all embryos from treated and control groups were treated by liquid nitrogen grinding and then stored at −80° for genomic DNA extraction. Additionally, adult turtle testis was prepared and stored at −80° for Amh complementary DNA (cDNA) cloning. All animal experiments were carried out according to a protocol approved by Zhejiang Wanli University.
Cloning of P. sinensis Amh cDNA
The total RNA from testis of adult turtle P. sinensis was extracted using TRIzol reagent (Invitrogen). The first cDNA was then synthesized from 2 μg of RNA by using the RevertAid First Strand cDNA Synthesis Kit (Fermentas), following the manufacturer’s instructions. 5′ and 3′ RACE was carried out according to the manufacturer’s protocol for the SMART RACE cDNA Amplification Kit (Clontech, Takara). The sequences of primers for RACE are as follows: Amh-GSPF1: 5′-CGCTCTCCACCCGCATCCCCGACT-3′; Amh-GSPF2: 5′-GGTTTCTGCCTCGCTCTTCAGTCCT-3′; Amh-GSPR1: 5′-TACTGCAAAGCGACTC CTAGCAC-3′; Amh-GSPR2: 5′-TGGCAGACATTTCTCTTAGGGCTT-3′. The PCR products were extracted from agarose gel using MiniBEST Agarose Gel DNA Extraction Kit (Takara) based on manufacturer’s instructions, cloned into pMD18-T (Takara) vector, and then transformed into Escherichia coli DH5α for sequencing. Alignment of deducted amino acid sequences were carried out by Clustal X software, and the phylogenetic tree was constructed using the neighbor-joining method in Mega 6.0 software. The sequences of amino acid used in the phylogenetic analysis were obtained from GenBank (NCBI).
Aromatase inhibitor letrozole treatment
A nonsteroidal aromatase inhibitor (AI) letrozole (Sigma, St. Louis, MO) were administered to eggs at developmental stage 15 and 16 (gonadal differentiation normally begins from late stage 17). The letrozole was dissolved in 95% ethanol at a concentration of 20 μg/μl, and 10 μl of drug was topically applied to the eggshell in the region adjacent to the embryo. Controls were treated with 10 μl of 95% ethanol. Each 200 eggs, i.e., 200 eggs in the AI group and 200 eggs in the control group, were treated in AI-treated and control group. Gonads of every 30 embryos were separated from adjacent mesonephros at stages 17, 21, and 25, and stored for quantitative RT-PCR (qRT-PCR) analysis. GMCs from all embryos surviving until stage 27 (67 and 72 embryos in AI-treated and control group, respectively) were dissected for histology and immunohistochemistry.
Construction of LV-Amh-shRNA vector system
The lentivirus vector (LV) was used to deliver short hairpin RNAs (shRNAs) specifically targeting Amh messenger RNA (mRNA) into living embryos of Chinese soft-shelled turtle before sexual differentiation, to knockdown endogenous Amh transcripts. The designed shRNA construct contained a unique 21 nt double-stranded Amh sequence that presented as an inverted complementary repeat, a loop sequence (5′-TTCAAGAGA-3′), and the RNA Plo-II terminator (5′-TTTTTT-3′). The full sequence of shRNA is 5′-GGTGCTGCATCTTGAGGAAGTTTCAAGAGAACTTCCAATCAAG ATGCAGCACC TTTTTT-3′. Annealed oligonucleotides were ligated into pGP-U6 (GenePharma, Shanghai, China) between the Bbs and Xho sites by T4 DNA ligase (Takara) to produce pGP-U6-Amh-shRNA. The pGP-U6-Amh-shRNA construct was digested with AgeI-EcoRI and inserted into the EcoRI site of pGLV-U6-GFP (GenePharma). The LV can also express green fluorescent protein (GFP), providing rapid visual assessment of the viral infection efficiency of embryos. The recombinant vector pGLV-GFP-Amh-shRNA was termed as LV-Amh-shRNA. The negative control vector (pGLV-GFP-NC-shRNA, termed as LV-NC-shRNA) contained a nonsense shRNA insert to control any effects caused by non-RNA interference mechanisms. The details for shRNA construct and vector map are showed in Supplemental Material, Figure S1.
For the generation of lentivirus, 293 T producer cells were transfected with optimized packaging plasmids (pGag/Pol, pRev, and pVSV-G) along with pGLV-Amh-shRNA or pGLV-NC-shRNA expression clone constructs by lipofectamine. At 24 hr post transfection, the transfection mix was replaced by a fresh culture medium (without antibiotics). The virus-containing supernatant was harvested at 72 hr post transfection, cleared by centrifugation (3000 rpm/min, for 15 min, at 4°), and then filtered through a 0.45 μm filter (Millipore, Bedford, MA). Viruses were titrated by adding serial dilutions to fresh 293 T and assessed using GFP expression after 48 hr. Viral titers of ∼1 × 109 infectious units/ml were obtained. Lentivirus aliquots were stored at −80° before infection of turtle embryos.
Construction of LV-Amh-OE vector system
Total RNA was isolated from testis of adult Chinese soft-shelled turtle and then reverse transcription was performed to prepare the cDNA. The full-length open reading frame (1401 bp) of the P. sinensis Amh gene was PCR amplified from cDNA using forward primer 5′-CCCCAAATTGTAGAGGCGAACC-3′ and reverse primer 5′-TGAGGGCAGGGCAGAGGAGG-3′. The PCR product was digested with EcoRI and cloned to pGLV-EF1a-GFP (LV-4; GenePharma). The recombinant vector pGLV-GFP-Amh was named LV-Amh. The empty vector pGLV-GFP-empty was constructed as a negative control (LV-empty). High-quality proviral DNA was used to transfect 293 T cells. Virus propagation was carried out as described above.
Infection of turtle embryos
A high-titer virus of LV-Amh-shRNA or LV-Amh-OE (at least 1 × 108 infectious units/ml, 5 μl per embryo) was injected into turtle eggs in a region adjacent to the embryo at stage 14 before the time point (stage 15) that Amh began to exhibit a highly male (ZZ)-specific expression pattern, using a fine metal Hamilton needle (diameter: 0.5 mm). Each 200 eggs (200 eggs per group, including two treated groups and two control groups) were injected in two treated groups, and 200 control eggs were injected with scrambled control virus of LV-NC-shRNA or LV-empty.
Eggs were sealed with parafilm and incubated for the indicated time points (stages 25 and 27). All embryos surviving until stage 25 were dissected for sex reversal analysis, except for 20 embryos left for examining tails at stage 27 in each group. Specifically, one of a pair of GMCs from every living embryo was examined by histology and immunostaining, and the other one was used for RNA isolation and then qRT-PCR analysis.
Embryo sexing
The genomic DNA was extracted from all tested embryos, and amplification of sex chromosome-specific DNA fragment was subsequently performed to identify the genetic sex of each embryo, which has been well documented (Literman et al. 2017). PCR products were visualized on 1% agarose gels. The lower bands represent Z-linked amplified fragments, and higher bands represent W-linked sex-diagnostic fragments (Figure S2). The primer sequences for PCR are as follows: Setd1b (forward: 5′-GATCGAATTACATCCTGC CT-3′; reverse: 5′-TAAATTAG GACTGGAAGACACC-3′).
qRT-PCR
Total RNA was extracted from embryonic gonads of different developmental stages, and subsequently synthesized for cDNA (methodology found above). Quantification of gene transcript levels in embryonic gonads of all treated and control groups was measured by qRT-RCR. In all PCR reactions, Gapdh was used as a reference gene. The qRT-RCR reaction was carried out using SYBR PrimeScript II (Takara) in a Bio-Rad iCycler system. After normalization with Gapdh, relative RNA levels in samples were calculated using the comparative threshold cycle method. At least three biological replicates were performed in each experiment, and each RNA sample was analyzed in triplicate determinations. The primers sequences for PCR are as follows: Gapdh (forward: 5′-GGC TTT CCG TGT TCC AAC TC-3′; reverse: 5′-GAC AAC CTG GTC CTC CGT GTA TC-3′), Amh (forward: 5′-CGG CTA CTC CCA CAC G-3′; reverse: 5′-CCT GGC TGG AGT ATT TGA CGG-3′), Cyp19a1 (forward: 5′-TCG TGG CTG TAC AAG AAA TAC GAA-3′; reverse: 5′-CCA GTC ATA TCT CCA CGG CTC T-3′), and Sox9 (forward: 5′-TTT CCG ACC GCT AAA ACG ACA C-3′; reverse: 5′-CTC CGC TGA CCA AAA CTT AGC CC-3′).
Immunofluorescence
GMCs were fixed in 4% paraformaldehyde overnight at 4°, dehydrated in graded ethanol, then embedded in paraffin wax and sectioned. Paraffin sections (5–6 μm) were deparaffinized and rehydrated before immersion in 10 mM sodium citrate buffer for 20 min at a subboiling temperature (96–99°) for antigen retrieval. After blocking for 1 hr in blocking solution (10% normal donkey serum, 3% BSA, and 0.3% Triton X-100) at room temperature, sections were covered with primary antibodies and incubated overnight at 4°, followed by washing (three times, 10 min each time) in washing solution (1% normal donkey serum, 3% BSA, 0.3% Triton X-100), incubation with secondary antibodies (2 hr, room temperature, dark environment), and further washing (same as above). The primary antibodies used in this analysis included rabbit anti-AMH (1:200, produced privately through Sangon Biotech, the specific sequence of the epitope used for AMH antibody production: PLGSGGSDPTELPRGHRKLRTLLLLKALQTVRAH WQEKRKVSRQNRSAGSQAYCRLQELTI NLRYDFIIVPVEYTANNCEGPCRRPLSTRSPGYSSHTVLLLEMQARGTPLRRSPCCVPVKYSSQDVILFTGEGLQVTTYPEMVVEECGCR), rabbit anti-VASA (1:500; Abcam), rabbit anti-SOX9 (1:500; Millipore), and mouse anti-CTNNB1 (1:250; Sigma). Primary antibodies were detected using secondary antibodies Alexa Fluor 488 donkey anti-rabbit IgG or Alexa Fluor 594 donkey anti-rabbit IgG, and Alexa Fluor 488 donkey anti-mouse IgG (1:250; Invitrogen). Nuclei were stained with DAPI (286 nmol/liter; Sigma) and then washed with 0.01 mol/liter PBS (three times, 5 min each time). Fluorescence signals were observed under a fluorescence microscope (Ti-E; Nickon, Garden City, NY) or confocal microscope (A1 Plus; Nickon).
Statistical analyses
Each experiment was independently performed based on at least three biological replicates (n ≥ 3). All data were expressed as the means ± SD and analyzed by one-way Duncan test and ANOVA using the SPSS software. For all analyses, a P-value < 0.05 was regarded as statistically significant (* P < 0.05, ** P < 0.01, *** P < 0.001).
Data availability
All data necessary for confirming the conclusions in this paper are included in this article and accompanying figures and tables. Supplemental material available at figshare: https://doi.org/10.25386/genetics.10028399.
Results
Characterization of Amh gene in P. sinensis
The full-length coding sequence of P. sinensis Amh was obtained by 5′ and 3′ RACE. The complete cDNA sequence of P. sinensis Amh was 3232 bp (accession number KY964412), with a 997 bp 5′ untranslated region, an open reading frame of 1401 bp, and an 834 bp 3′ untranslated region (Figure S3A). The deduced AMH protein comprised 466 amino acids, which includes two characteristic functional domains of the TGF-β superfamily: AMH-N and TGF-β domain with ten canonical cysteine residues. The amino acid sequence of P. sinensis AMH shared 47%, 32.22%, 19.25%, 23.77%, 19.42%, 21.06%, 18.55%, 11.30%, and 15.38% identity with that of the red-eared slider turtle (Trachemys scripta), chicken (Gallus gallus), mouse (Mus musculus), coelacanthidae (Latimeria chalumnae), spotted gar (Lepisosteus oculatus), human (Homo sapiens), frog (Xenopus laevis), zebrafish (Danio rerio), and elephant shark (Callorhinchus milii), respectively (Figure S3B). The phylogenetic tree also showed that P. sinensis AMH was evolutionarily most closely related to the red-eared slider turtle, followed by chicken and mouse, and distantly related to fish (Figure S3C).
Sexually dimorphic expression of Amh in gonads of P. sinensis
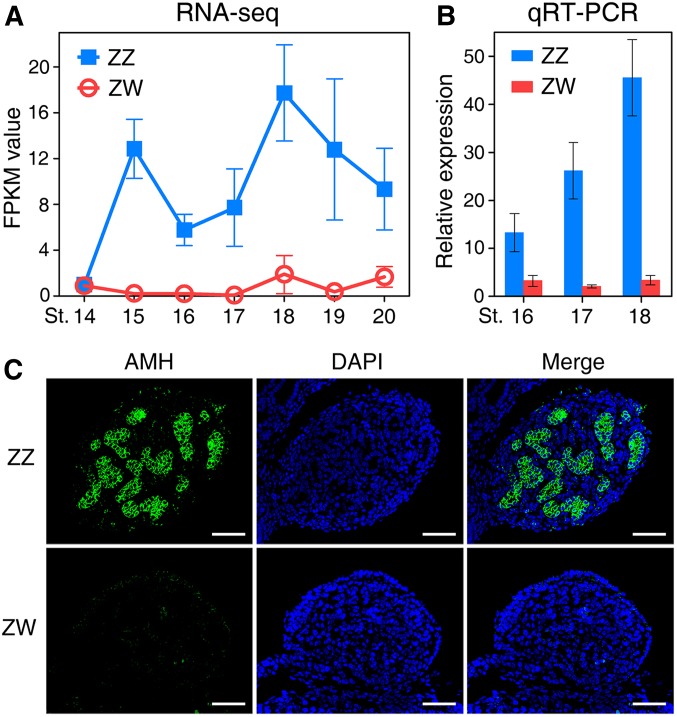
To find out whether Amh is involved in testicular development in P. sinensis, we first analyzed the expression profile of Amh in embryonic gonads of both sexes at different developmental stages. Detailed staging criteria for P. sinensis has been described for embryos between late neurula (stage 5) and hatching (stage 27), where staging was based primarily on days of incubation (Tokita and Kurartani 2001). Our analysis covered stages between late somite stage (stage 14, 14 days of incubation) to hatching (stage 27, 48 days of incubation). See Materials and Methods for the specific stages analyzed, with their corresponding days of incubation. RNA-sequencing showed that Amh transcripts were detected and already expressed highly in the male gonads as early as stage 15. It exhibited male-specific embryonic expression during the critical sex determination period (stages 15–19), with female gonads showing extremely low expression level (Figure 1A). The sex-dependent expression was further confirmed by qRT-PCR (Figure 1B). We also examined the cellular localization of AMH protein in embryonic gonads at stage 17, when the gonads were still morphologically undifferentiated and appeared identical between sexes. Immunofluorescence showed that AMH protein was robustly expressed in Sertoli cells of the medullary sex cords in male embryonic gonads, whereas the expression signals were undetectable in female gonads (Figure 1C).
Figure 1.
The sexually dimorphic expression of Amh in early embryonic gonads of P. sinensis. (A and B) The transcript expression levels of Amh in gonads of both sexes during the critical sex determination period (stages 15–19), determined by RNA-sequencing (A) and qRT-PCR (B). Amh exhibited a highly male-specific expression pattern in early embryonic gonads. Data are shown as means ± SD. N ≥ 3. (C) Immunofluorescence of AMH in male and female embryonic gonads at stage 17. AMH protein was robustly expressed in the medullary region of ZZ gonads. Bar, 50 μm.
Upregulation of Amh in ZW gonads during female-to-male sex reversal
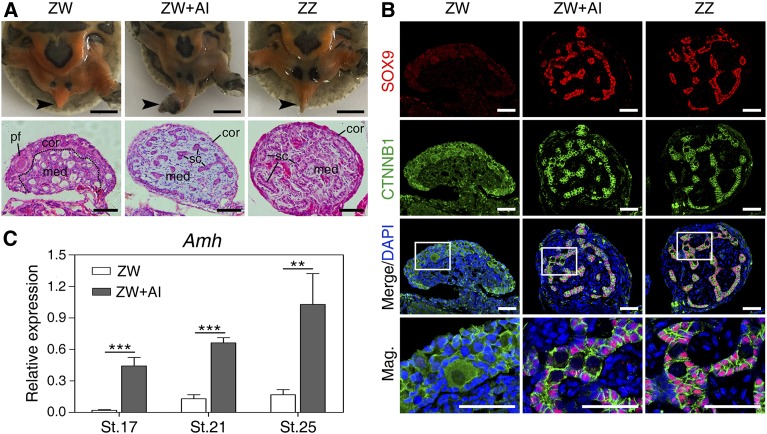
Treatment of AI letrozole at early stages of sex determination (stages 15 and 16) induced ZW turtle embryos to develop toward the male phenotype (Figure 2A). Tails of control ZW embryos hid under the calipash, shorter than those of control ZZ embryos. However, tails of AI-treated ZW embryos became longer, with male genitals exposed from the cloacal orifice in most cases. The gonadal histological analysis showed that AI-treated ZW embryos exhibited medullary testis cords and degenerated cortex (Figure 2A). Furthermore, the testicular marker SOX9 was induced to be robustly expressed in medulla of the masculinized ZW gonads (Figure 2B). These observations demonstrated that AI treatment at early stages indeed induced female-to-male sex reversal in P. sinensis.
Figure 2.
Upregulation of Amh in masculinized ZW gonads induced by aromatase inhibitor (AI). (A) Tail morphology (black arrow) and hematoxylin and eosin (H&E) staining of gonadal sections from control ZW, ZW + AI, and control ZZ P. sinensis of stage 27. The male-to-female sex reversal were observed in ZW + AI gonads characterized by morphologically altered tail and medullary sex-cord formation. Bar, 5 mm and 50 μm, respectively. (B) Double immunofluorescence of SOX9 and CTNNB1 in gonadal sections of control ZW, ZW + AI, and control ZZ P. sinensis of stage 27. Ectopic expression of SOX9 protein were activated in masculinized medulla of ZW gonads. Bar, 50 μm. (C) The mRNA expression of Amh in ZW gonads with AI treatment at stage 17, 21, and 25, showing rapid and remarkable upregulation, determined by qRT-PCR analysis. Data are shown as means ± SD. N ≥ 3. ** P < 0.01; *** P < 0.001. cor, cortical region; med, medullary region; pf, primordial follicle; sc, Sertoli cell.
We next analyzed the expression changes of Amh in AI-induced female-to-male sex reversal to further determine whether Amh expression is associated with the testicular differentiation. qRT-PCR showed that Amh expression in ZW gonads increased dramatically in response to the female-to-male sex reversal (Figure 2C). Intriguingly, the upregulation of Amh responded as early as stage 17 (Figure 2C), when the gonads were still morphologically undifferentiated between sexes, indicating that Amh is an early responder to the induction of male differentiation in P. sinensis.
Feminization of ZZ turtle embryos with Amh knockdown
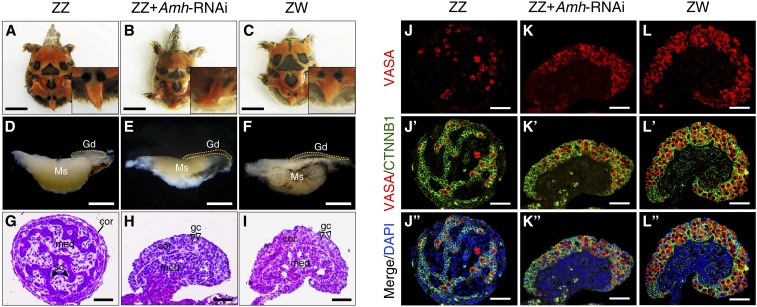
To investigate the function of Amh on male development of P. sinensis, we first established the Amh-deficient turtle model by introducing shRNA against Amh in ovo at stage 14. qRT-PCR showed that the mRNA expression of Amh was >80% decreased in ZZ gonads from the embryos exhibiting global GFP reporter expression after LV-Amh-shRNA treatment compared with control ZZ gonads (LV-NC-shRNA) (Figure S4, A and B). Phenotype of Amh-deficient ZZ gonads were subsequently examined by gonadal histology and immunofluorescence. Control ZZ embryonic tails were straight and beyond the hem of the calipash, but ZW embryonic tails were relative shorter and hid under the calipash (Figure 3, A and C). Control ZZ gonads were short and cylindrical, while ZW gonads were long and flat (Figure 3, D and F). In ZZ embryos with Amh knockdown, tails became curved and did not exceed the hem of the calipash, and gonads became elongated and flat, exhibiting female-like morphology (Figure 3, B and E). Histological analysis of gonadal sections showed that the control ZZ gonads of stage 25 possessed a dense medulla, with seminiferous cords and a degenerative cortex (Figure 3G). However, a vacuolated medulla and a well-developed outer cortex were clearly observed in control ZW gonads (Figure 3I). However, the Amh-deficient ZZ gonads were completely feminized, characterized by a thickened cortex and a highly degenerated medulla (Figure 3H). VASA staining showed that germ cells mainly located in medullary cords of control ZZ gonads, whereas control ZW gonads exhibited outer cortical distribution pattern of germ cells (Figure 3, J and L). VASA-positive germ cells in Amh-deficient ZZ gonads displayed a female-like distribution, mainly enriched in the thickened cortex (Figure 3K). Statistical analysis showed that 32.8% (21 of 64) of genetic male embryos with LV-Amh-shRNA treatment appeared male-to-female sex reversal [genetic male (ZZ) embryos differentiate towards females] (Table 1, all live embryos, regardless of GFP expression, were included in the analysis).
Figure 3.
Feminization of ZZ embryos following Amh knockdown in ovo. (A–C) Morphology of tails from control ZZ, ZZ + Amh-RNAi, and control ZW P. sinensis of stage 27. Bar, 1 cm. (D–F) Representative images of the gonad-mesonephros complexes (GMCs) from control ZZ, ZZ + Amh-RNAi, and control ZW embryos of stage 25. The ZZ gonads with Amh knockdown became elongated and flat compared to control ZZ gonads. Gonads were outlined by yellow dotted lines. Bar, 1 mm. (G–I) H&E staining of gonadal sections from control ZZ, ZZ + Amh-RNAi, and control ZW embryos of stage 25. The ZZ gonads with Amh knockdown appeared thickened outer cortex and degenerated testis cord in medullary region, similar to control ZW gonads. The white dotted lines showed the separation between cortical and medullar regions. Bar, 50 μm. (J–L’’) VASA and CTNNB1 immunostaining of gonadal sections from control ZZ, ZZ + Amh-RNAi and control ZW embryos of stage 25. A female-typical distribution pattern of germ cells was observed in Amh-deficient ZZ gonads. Bar, 50 μm. cor, cortical region; gc, germ cells; Gd, gonad; H&E, hematoxylin and eosin; med, medullary region; Ms, mesonephros; RNAi, RNA interference; sc, Sertoli cell.
Table 1. Phenotypes of embryos after LV-Amh-shRNA or LV-Amh-OE treatment.
| Viral treatment | No. of injected embryos | Embryos surviving until stage 25 | Genotype of embryos | Phenotype of embryos | Sex reversal ratea |
|---|---|---|---|---|---|
| LV-NC-shRNA | 200 | 175 | ZZ:88; ZW:87 | M:88; F:87 | 0/88 |
| LV-Amh-shRNA | 200 | 138 | ZZ:64; ZW:74 | M:43; F:95 | 21/64 |
| LV-empty | 200 | 150 | ZZ:78; ZW:72 | M:78; F:72 | 0/72 |
| LV-Amh-OE | 200 | 124 | ZZ:62; ZW:62 | M:78; F:46 | 16/62 |
Genotype of embryos was identified by amplification of sex chromosome-specific DNA fragment. Phenotype of embryos was assessed by gonadal histology and SOX9 immunofluorescence.
Male-to-female sex reversal rate was determined by the number of feminized genetic male embryos divided by the total number of ZZ embryos; female-to-male sex reversal rate was determined by the number of masculinized genetic female embryos divided by the total number of ZW embryos.
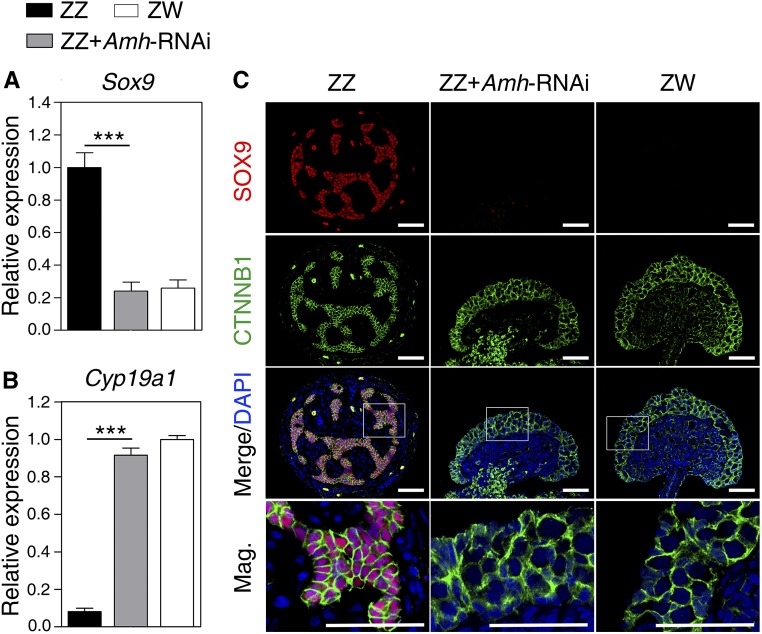
To further confirm the activation of the female developmental pathway in Amh-deficient ZZ embryos, we analyzed the expression changes of testicular differentiation marker Sox9 and ovarian development regulator Cyp19a1. At the mRNA level, significant downregulation of Sox9 and remarkable upregulation of Cyp19a1 were observed in ZZ gonads of stage 25 with Amh knockdown, relative to controls (Figure 4, A and B). At the protein level, the expression signals of SOX9 was detected specifically in the nuclei of Sertoli cells in control ZZ gonads, but it was not observed in control ZW gonads. SOX9 expression in Amh-deficient ZZ gonads was sharply reduced and almost disappeared (Figure 4C). These results suggested that loss of Amh in ZZ turtle embryos led to male-to-female sex reversal.
Figure 4.
The Sox9 and Cyp19a1 expression change in response to Amh knockdown. (A and B) qRT-PCR of Sox9 and Cyp19a1 in control ZZ, ZZ + Amh-RNAi, and control ZW gonads of stage 25, showing significantly reduced Sox9 expression and increased Cyp19a1 expression in Amh-deficient ZZ gonads. Data are shown as means ± SD. N ≥ 3. *** P < 0.001. (C) Double immunofluorescence of SOX9 and CTNNB1 in sections of control ZZ, ZZ + Amh-RNAi, and control ZW gonads of stage 25. SOX9 protein expression almost disappeared in Amh-deficient ZZ gonads. Bar, 50 μm. RNAi, RNA interference.
Masculinization of ZW turtle embryos overexpressing Amh
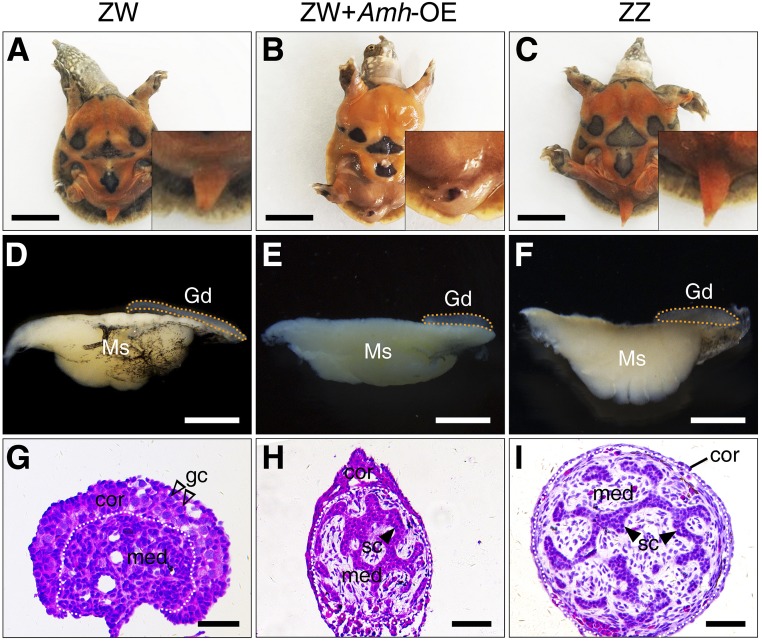
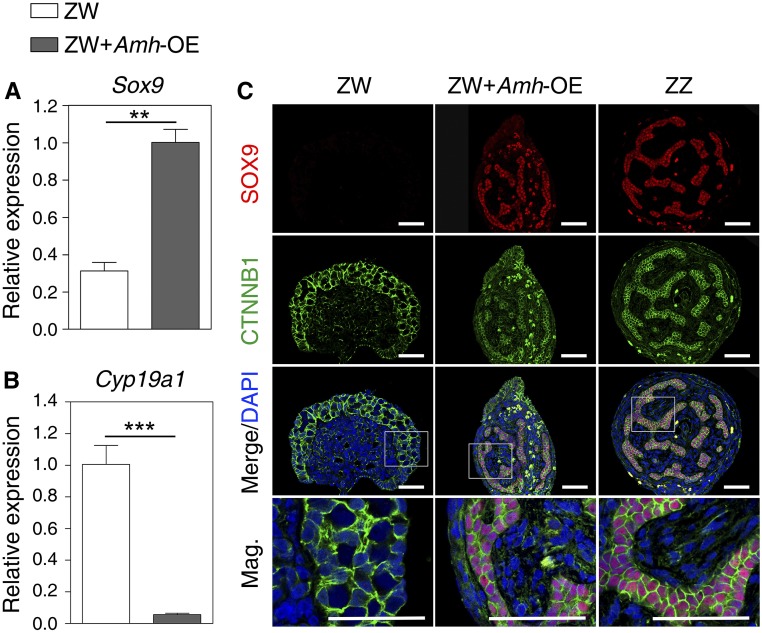
The ectopic expression of Amh in ZW embryos was performed to determine if Amh was sufficient to initiate primary male differentiation in P. sinensis. Amh-overexpressing embryos were generated by injection of LV carrying the Amh open reading frame into turtle eggs at stage 14 (Figure S4C). In ZW embryos overexpressing Amh, the tails became curved and the gonads exhibited a short cylindrical structure, similar to control ZZ gonads (Figure 5, A–F). Hematoxylin and eosin staining of gonadal sections showed that ZW gonads overexpressing Amh exhibited a well-developed medulla with seminiferous cord-like structure (Figure 5, G–I). In the LV-Amh-OE–treated group, 25.8% (16 of 62) of ZW embryos showed a female-to-male sex reversal (Table 1). Upregulation of Sox9 and downregulation of Cyp19a1 were observed in ZW gonads with Amh overexpression, determined by qRT-PCR (Figure 6, A and B). Ectopic activation of SOX9 protein in treated ZW gonads was further confirmed by immunofluorescence. Induced SOX9 expression was localized in the nuclei of Sertoli cells within the masculinized region (testis cords) in ZW gonads following Amh overexpression, but it seemed a little bit lower compared to control males (Figure 6C). These data indicated that overexpression of Amh caused obvious masculinization of genetic female (ZW) embryos in P. sinensis.
Figure 5.
Masculinization of ZW embryos overexpressing Amh in ovo. The tails (A–C), GMCs (D–F), and H&E staining of gonadal sections (G–I) from control ZW, ZW + Amh-OE, and control ZZ embryos. The ZW embryos overexpressing Amh showed the female-to-male sex reversal, characterized by curved tails and male-like gonads with seminiferous cord-like structure in medulla. Gonads were outlined by yellow dotted lines. Bar, 1 cm (A–C), 1 mm (D–F), and 50 μm (G–I), respectively. cor, cortical region; gc, germ cells; Gd, gonad; med, medullary region; Ms, mesonephros; sc, Sertoli cell.
Figure 6.
The Sox9 and Cyp19a1 expression change in response to Amh overexpression. (A and B) qRT-PCR of Sox9 and Cyp19a1 in control ZW, ZW + Amh-OE, and control ZZ gonads of stage 25, showing increased Sox9 expression and reduced Cyp19a1 expression in ZW gonads overexpressing Amh. Data are shown as means ± SD. N ≥ 3. ** P < 0.01; *** P < 0.001. (C) Double immunofluorescence of SOX9 and CTNNB1 in sections of control ZW, ZW + Amh-OE, and control ZZ gonads of stage 25. SOX9 protein was induced to express robustly in gonadal medulla of ZW embryos overexpressing Amh. Bar, 50 μm.
Discussion
The conserved roles of TGF-β signaling pathway in sex determination have been recently functionally characterized in teleost fish, through the discoveries of three sex-determining genes, Amhr2, Gsdf, and Amhy (Hattori et al. 2012; Kamiya et al. 2012; Myosho et al. 2012). In this study, we provide the first solid evidence that Amh is both necessary and sufficient to induce male development in a reptilian species, P. sinensis, highlighting the significance of the TGF-β pathway in reptilian sex determination and sexual differentiation.
In this study, we found that the male gonad-specific expression of P. sinensis Amh has already appeared as early as stage 15, clearly preceding the onset of gonadal differentiation, indicating an upstream role of Amh in the male pathway of P. sinensis. This finding is consistent with previous studies in the red-eared slider turtle (Shoemaker et al. 2007), painted turtle (Radhakrishnan et al. 2017), and American alligator (Western et al. 1999). In T. scripta with temperature-dependent sex determination, Amh expression in gonad was significantly higher at male- than female-producing temperature from stage 16 onward, the beginning of the temperature-sensitive sex determination period (Shoemaker et al. 2007; Shoemaker-Daly et al. 2010; Czerwinski et al. 2016). These correlative studies strongly imply the conserved role of Amh in male development across reptilian species.
In nonmammalian vertebrates, estrogen and its synthetase aromatase play an important regulatory role in early gonadal sex differentiation. Exogenous estrogen and AI can override the effects of primary sex-determination signals, including genetic and environmental factors, if applied during critical developmental periods (Crews 1994a,b; Smith et al. 2003; Schulz et al. 2007; Kobayashi et al. 2008; Ge et al. 2017). Treatment of AI into chicken ZW eggs was able to induce upregulation of Dmrt1, a Z chromosome-linked master sex-determining gene, ultimately resulting in female-to-male sex reversal (Smith et al. 2003). It has been proposed that exogenous steroid hormones may redirect the differentiation direction of gonads by interacting with the sex-specific genes, especially those located on the upstream of sexual development pathway (Matsumoto and Crews 2012). In this study, Amh expression in P. sinensis ZW gonads responded rapidly to the AI-induced female-to-male sex reversal, before the sexual differentiation. The finding is consistent with the studies on zebrafish that reported the estrogen-induced alteration in Amh expression had already appeared at early stages of gonadal differentiation (Schulz et al. 2007). These observations suggest that Amh is associated with testicular differentiation, and likely lies on the upstream of male pathway in P. sinensis.
To date, any member of TGF-β signaling pathway has not been functionally identified in reptiles, including turtles. Using an in ovo turtle gene-modulating approach developed previously (Ge et al. 2017; Sun et al. 2017), we found that knockdown of Amh led to complete feminization of genetic male (ZZ) gonads, including gonadal morphology and germ cell distribution pattern, as well as downregulation of testicular marker Sox9 and upregulation of ovarian regulator Cyp19a1, indicating that Amh gene is essential for male gonadal differentiation in P. sinensis. This is similar to the functional roles of Amhy, the Y chromosome-linked duplicated copy of Amh, in two teleost fish (Hattori et al. 2012; Li et al. 2015). In Patagonian pejerrey, Amhy knockdown in XY embryos caused upregulation of Cyp19a1a and development of ovaries (Hattori et al. 2012). Likewise, knockdown of Amhy in XY Nile tilapia resulted in ovarian differentiation (Li et al. 2015). Conversely, ectopic expression of Amh in P. sinensis ZW gonads induced the formation of sex cord-like structures with robust expression of SOX9 protein, implying that Amh is sufficient to initiate testicular differentiation in P. sinensis. As expected, the genetic female (XX) gonads overexpressing Amhy developed into testis in Nile tilapia (Li et al. 2015). Recently, we found the same loss- and gain-of-function role of Amh in T. scripta, a turtle species with temperature-dependent sex determination (data not published), suggesting a conserved role for Amh in sex determination of turtle species, even with different sex determination systems. Our previous studies on P. sinensis have reported that the onset of Amh expression preceded Sox9, but later than Dmrt1, and Dmrt1 overexpression caused an elevated expression of Amh and Sox9 in ZW P. sinensis (Sun et al. 2017). In this study, ectopic activation of Sox9 occurred in response to Amh overexpression, which means that Amh could regulate Sox9 in P. sinensis. Amh expression was also earlier than Sox9 in chicken (Oreal et al. 1998) and American alligators (Western et al. 1999); however, the genetic position between Amh and Sox9 was opposite in mammals (Arango et al. 1999). All these findings indicate that P. sinensis Amh acts as a positive regulator in the primary male sexual differentiation, and the network of Dmrt1-Amh-Sox9 might be the effective component of testicular development in P. sinensis. Despite the necessary and sufficient role, Amh and Dmrt1 do not seem to be the master sex-determining gene because neither gene localizes on the sex chromosome. Further investigation will be required to identify the master sex-determining gene in P. sinensis. Understanding the genetic link between the putative master gene and male or female effective components (such as Amh) may finally unravel the full mechanism of sex determination and differentiation in P. sinensis.
In conclusion, we demonstrate for the first time in reptiles that Amh is both necessary and sufficient to drive testicular development, thereby operating as an upstream regulator in the male pathway of the Chinese soft-shelled turtle P. sinensis. This study highlights a conserved role of a member of TGF-β signaling pathway, Amh, in reptilian sex determination and gonadal differentiation, but the direct upstream regulator of Amh remains to be identified.
Acknowledgments
We thank Mr. Wei Song and Caisheng Wang for turtle eggs collection and incubation. This study was supported by the National Natural Science Foundation of China (31922084, 31872960), National Key Research and Development Program (2018YFD0900203), Natural Science Foundation of Zhejiang Province for Distinguished Young Scholars (LR19C190001), the Basic Public Welfare Research Projects of Zhejiang Province (LGN19C190005), the Major Agricultural Project of Ningbo (2017C110012), the Zhejiang Provincial Project of Selective Breeding of Aquatic New Varieties (2016C02055-4), and Zhejiang Provincial Top Key Discipline of Biological Engineering (KF2016005, ZS2018008).
Author contributions: C.G. and G.Q. conceived and designed the study; Y.Z., W.S., H.C., H.B., and Y.Z. performed the experiments; Y.Z. and W.S. analyzed data; and Y.Z., W.S., and C.G. cowrote the manuscript. All authors read and approved the manuscript.
Footnotes
Supplemental material available at https://doi.org/10.25386/genetics.10028399.
Communicating editor: B. Draper
Literature Cited
- Arango N. A., Lovell-Badge R., and Behringer R. R., 1999. Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell 99: 409–419. 10.1016/S0092-8674(00)81527-5 [DOI] [PubMed] [Google Scholar]
- Boulanger L., Pannetier M., Gall L., Allais-Bonnet A., Elzaiat M. et al. , 2014. FOXL2 is a female sex-determining gene in the goat. Curr. Biol. 24: 404–408. 10.1016/j.cub.2013.12.039 [DOI] [PubMed] [Google Scholar]
- Chen S., Zhang G., Shao C., Huang Q., Liu G. et al. , 2014. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat. Genet. 46: 253–260. 10.1038/ng.2890 [DOI] [PubMed] [Google Scholar]
- Crews D., 1994. Temperature, steroids and sex determination. J. Endocrinol. 142: 1–8. 10.1677/joe.0.1420001 [DOI] [PubMed] [Google Scholar]
- Crews D., and Bergeron J. M., 1994. Role of reductase and aromatase in sex determination in the red-eared slider (Trachemys scripta), a turtle with temperature-dependent sex determination. J. Endocrinol. 143: 279–289. 10.1677/joe.0.1430279 [DOI] [PubMed] [Google Scholar]
- Czerwinski M., Natarajan A., Barske L., Looger L. L., and Capel B., 2016. A timecourse analysis of systemic and gonadal effects of temperature on sexual development of the red-eared slider turtle Trachemys scripta elegans. Dev. Biol. 420: 166–177. 10.1016/j.ydbio.2016.09.018 [DOI] [PubMed] [Google Scholar]
- Eshel O., Shirak A., Dor L., Band M., Zak T. et al. , 2014. Identification of male-specific amh duplication, sexually differentially expressed genes and microRNAs at early embryonic development of Nile tilapia (Oreochromis niloticus). BMC Genomics 15: 774 10.1186/1471-2164-15-774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C., Ye J., Zhang Y., Sun W., Sang Y. et al. , 2017. Dmrt1 induces the male pathway in a turtle species with temperature-dependent sex determination. Development 144: 2222–2233. 10.1242/dev.152033 [DOI] [PubMed] [Google Scholar]
- Hattori R. S., Murai Y., Oura M., Masuda S., Majihi S. K. et al. , 2012. A Y-linked anti-Mullerian hormone duplication takes over a critical role in sex determination. Proc. Natl. Acad. Sci. USA 109: 2955–2959. 10.1073/pnas.1018392109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., Kent T. R., Urick M. E., and Giles J. R., 2008. Expression and regulation of anti-Müllerian hormone in an oviparous species, the hen. Biol. Reprod. 78: 13–19. 10.1095/biolreprod.107.061879 [DOI] [PubMed] [Google Scholar]
- Josso N., di Clemente N., and Gouédard L., 2001. Anti-Müllerian hormone and its receptors. Mol. Cell. Endocrinol. 179: 25–32. 10.1016/S0303-7207(01)00467-1 [DOI] [PubMed] [Google Scholar]
- Kamiya T., Kai W., Tasumi S., Oka A., Matsunaga T. et al. , 2012. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (fugu). PLoS Genet. 8: e1002798 10.1371/journal.pgen.1002798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T. R., Lee B. K., Behringer R. R., and Eicher E. M., 1991. Mapping anti-Müllerian hormone (amh) and related sequences in the mouse: identification of a new region of homology between MMU10 and HSA19p. Genomics 11: 273–283. 10.1016/0888-7543(91)90133-Y [DOI] [PubMed] [Google Scholar]
- Klüver N., Pfennig F., Pala I., Storch K., Schlieder M. et al. , 2007. Differential expression of anti-Müllerian hormone (amh) and anti-Müllerian hormone receptor type II (amhrII) in the teleost medaka. Dev. Dyn. 236: 271–281. 10.1002/dvdy.20997 [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Kajiura-Kobayashi H., Guan G., and Nagahama Y., 2008. Sexual dimorphic expression of DMRT1 and Sox9a during gonadal differentiation and hormone-induced sex reversal in the teleost fish Nile tilapia (Oreochromis niloticus). Dev. Dyn. 237: 297–306. 10.1002/dvdy.21409 [DOI] [PubMed] [Google Scholar]
- Koopman P., Münsterberg A., Capel B., Vivian N., and Lovell-Badge R., 1990. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature 348: 450–452. 10.1038/348450a0 [DOI] [PubMed] [Google Scholar]
- Koopman P., Gubbay J., Vivian N., Goodfellow P., and Lovell-Badge R., 1991. Male development of chromosomally female mice transgenic for Sry. Nature 351: 117–121. 10.1038/351117a0 [DOI] [PubMed] [Google Scholar]
- Lambeth L. S., Raymond C. S., Roeszler K. N., Kuroiwa A., Nakata T. et al. , 2014. Over-expression of DMRT1 induces the male pathway in embryonic chicken gonads. Dev. Biol. 389: 160–172. 10.1016/j.ydbio.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth L. S., Ayers K., Cutting A. D., Doran T. J., Sinclair A. H. et al. , 2015. Anti-Müllerian hormone is required for chicken embryonic urogenital system growth but not sexual differentiation. Biol. Reprod. 93: 138 10.1095/biolreprod.115.131664 [DOI] [PubMed] [Google Scholar]
- Li M., Sun Y., Zhao J., Shi H., Zeng S. et al. , 2015. A tandem duplicate of anti-Müllerian hormone with a missense SNP on the Y chromosome is essential for male sex determination in Nile Tilapia, Oreochromis niloticus. PLoS Genet. 11: e1005678 10.1371/journal.pgen.1005678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Literman R., Radhakrishnan S., Tamplin J., Burke R., Dresser C. et al. , 2017. Development of sexing primers in Glyptemys insculpta and Apalone spinifera turtles uncovers an XX/XY sex-determining system in the critically-endangered bog turtle Glyptemys muhlenbergii. Conserv. Genet. Resour. 9: 651–658. 10.1007/s12686-017-0711-7 [DOI] [Google Scholar]
- Matsuda M., Nagahama Y., Shinomiya A., Sato T., Matsuda C. et al. , 2002. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 4l7: 559–563. 10.1038/nature751 [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., and Crews D., 2012. Molecular mechanisms of temperature-dependent sex determination in the context of ecological developmental biology. Mol. Cell. Endocrinol. 354: 103–110. 10.1016/j.mce.2011.10.012 [DOI] [PubMed] [Google Scholar]
- Miura T., Miura C., Konda Y., and Yamauchi K., 2002. Spermatogenesis-preventing substance in Japanese eel. Development 129: 2689–2697. [DOI] [PubMed] [Google Scholar]
- Myosho T., Otake H., Masuyama H., Matsuda M., Kuroki Y. et al. , 2012. Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics 191: 163–170. 10.1534/genetics.111.137497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda I., Kondo M., Hornung U., Asakawa S., Winkler C. et al. , 2002. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc. Natl. Acad. Sci. USA 99: 11778–11783. 10.1073/pnas.182314699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper M., Lowe R., Galuska S., Hofmann K. J., Smith R. G. et al. , 1996. Molecular cloning of an avian anti-Müllerian hormone homologue. Gene 176: 203–209. 10.1016/0378-1119(96)00248-X [DOI] [PubMed] [Google Scholar]
- Oreal E., Pieau C., Mattei M. G., Josso N., Picard J. Y. et al. , 1998. Early expression of AMH in chicken embryonic gonads precedes testicular SOX9 expression. Dev. Dyn. 212: 522–532. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan S., Literman R., Neuwald J., Severin A., and Valenzuela N., 2017. Transcriptomic responses to environmental temperature by turtles with temperature-dependent and genotypic sex determination assessed by RNAseq inform the genetic architecture of embryonic gonadal development. PLoS One 12: e0172044 10.1371/journal.pone.0172044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichwald K., Petzold A., Koch P., Downie B. R., Hartmann N. et al. , 2015. Insights into sex chromosome evolution and aging from the genome of a short-lived fish. Cell 163: 1527–1538. 10.1016/j.cell.2015.10.071 [DOI] [PubMed] [Google Scholar]
- Rey R., Lukas-Croisier C., Lasala C., and Bedecarrás P., 2003. AMH/MIS: what we know already about the gene, the protein and its regulation. Mol. Cell. Endocrinol. 211: 21–31. 10.1016/j.mce.2003.09.007 [DOI] [PubMed] [Google Scholar]
- Schulz R. W., Bogerd J., Male R., Ball J., Fenske M. et al. , 2007. Estrogen induced alterations in amh and dmrt1 expression signal for disruption in male sexual development in the zebrafish. Environ. Sci. Technol. 41: 6305–6310. 10.1021/es070785+ [DOI] [PubMed] [Google Scholar]
- Shirak A., Seroussi E., Cnaani A., Howe A. E., Domokhovsky R. et al. , 2006. Amh and Dmrta2 genes map to tilapia (Oreochromis spp.) linkage group 23 within quantitative trait locus regions for sex determination. Genetics 174: 1573–1581. 10.1534/genetics.106.059030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker C., Ramsey M., Queen J., and Crews D., 2007. Expression of Sox9, Mis and Dmrt1 in the gonad of a species with temperature-dependent sex determination. Dev. Dyn. 236: 1055–1063. 10.1002/dvdy.21096 [DOI] [PubMed] [Google Scholar]
- Shoemaker-Daly C. M., Jackson K., Yatsu R., Matsumoto Y., and Crews D., 2010. Genetic network underlying temperature- dependent sex determination is endogenously regulated by temperature in isolated cultured Trachemys scripta gonads. Dev. Dyn. 239: 1061–1075. 10.1002/dvdy.22266 [DOI] [PubMed] [Google Scholar]
- Sinclair A. H., Berta P., Palmer M. S., Hawkins J. R., Griffiths B. L. et al. , 1990. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346: 240–244. 10.1038/346240a0 [DOI] [PubMed] [Google Scholar]
- Smith C. A., Smith M. J., and Sinclair A. H., 1999. Gene expression during gonadogenesis in the chicken embryo. Gene 234: 395–402. 10.1016/S0378-1119(99)00179-1 [DOI] [PubMed] [Google Scholar]
- Smith C. A., Katz M., and Sinclair A. H., 2003. DMRT1 is upregulated in the gonads during female-to-male sex reversal in ZW chicken embryos. Biol. Reprod. 68: 560–570. 10.1095/biolreprod.102.007294 [DOI] [PubMed] [Google Scholar]
- Smith C. A., Roeszler K. N., Ohnesorg T., Cummins D. M., Farlie P. G. et al. , 2009. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 461: 267–271. 10.1038/nature08298 [DOI] [PubMed] [Google Scholar]
- Sun W., Cai H., Zhang G., Zhang H., Bao H. et al. , 2017. Dmrt1 is required for primary male sexual differentiation in Chinese soft-shelled turtle Pelodiscus sinensis. Sci. Rep. 7: 4433 [corrigenda: Sci. Rep. 8: 6322 (2018)] 10.1038/s41598-017-04938-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehana Y., Matsuda M., Myosho T., Suster M. L., Kawakami K. et al. , 2014. Co-option of Sox3 as the male-determining factor on the Y chromosome in the fish Oryzias dancena. Nat. Commun. 5: 4157 10.1038/ncomms5157 [DOI] [PubMed] [Google Scholar]
- Tokita M., and Kuratani S., 2001. Normal embryonic stages of the Chinese softshelled turtle Pelodiscus sinensis (Trionychidae). Zool. Sci. 18: 705–715. 10.2108/zsj.18.705 [DOI] [Google Scholar]
- Wang Z., Pascual-Anaya J., Zadissa A., Li W., Niimura Y. et al. , 2013. The draft genomes of soft-shell turtle and green sea turtle yield insights into the development and evolution of the turtle-specific body plan. Nat. Genet. 45: 701–706. 10.1038/ng.2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western P. S., Harry J. L., Graves J. A., and Sinclair A. H., 1999. Temperature-dependent sex determination in the American alligator: AMH precedes SOX9 expression. Dev. Dyn. 216: 411–419. [DOI] [PubMed] [Google Scholar]
- Wu G. C., Chiu P. C., Lyu Y. S., and Chang C. F., 2010. The expression of amh and amhr2 is associated with the development of gonadal tissue and sex change in the protandrous black porgy, Acanthopagrus schlegeli. Biol. Reprod. 83: 443–453. 10.1095/biolreprod.110.084681 [DOI] [PubMed] [Google Scholar]
- Yano A., Guyomard R., Nicol B., Jouanno E., Quillet E. et al. , 2012. An immune-related gene evolved into the master sex-determining gene in rainbow trout, Oncorhynchus mykiss. Curr. Biol. 22: 1423–1428. 10.1016/j.cub.2012.05.045 [DOI] [PubMed] [Google Scholar]
- Yoshimoto S., Okada E., Umemoto H., Tamura K., Uno Y. et al. , 2008. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proc. Natl. Acad. Sci. USA 105: 2469–2474. 10.1073/pnas.0712244105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga N., Shiraishi E., Yamamoto T., Iguchi T., Abe S. et al. , 2004. Sexually dimorphic expression of a teleost homologue of Müllerian inhibiting substance during gonadal sex differentiation in Japanese flounder, Paralichthys olivaceus. Biochem. Biophys. Res. Commun. 322: 508–513. 10.1016/j.bbrc.2004.07.162 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data necessary for confirming the conclusions in this paper are included in this article and accompanying figures and tables. Supplemental material available at figshare: https://doi.org/10.25386/genetics.10028399.