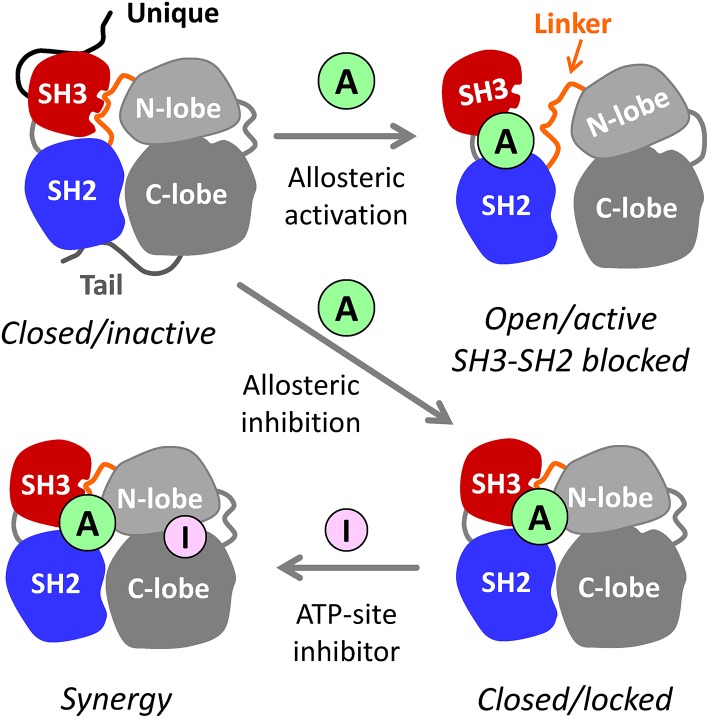
Figure 1.
Allosteric modulators of Src-family kinases. Hck, Fgr, and other Src-family kinases are regulated by intramolecular interactions of their SH3, SH2, and bi-lobed kinase domains (N- and C-lobes). The SH3 and SH2 domains pack against the back of the kinase domain to stabilize the inactive, assembled kinase conformation (upper left). Small molecule allosteric ligands (A) that bind to the SH3-SH2-linker region have the potential to disrupt its regulatory influence on the kinase domain, resulting in kinase activation (top right). Because SH3 and SH2 are also involved in trans-interactions with other proteins, small molecules that bind to this region may interfere with signaling by altering substrate recruitment. Allosteric ligands also have the potential to stabilize the closed, inactive conformation (lower right). Such compounds may inhibit the kinase directly and/or enhance the potency of existing ATP-site inhibitors (I) that prefer a particular conformation of the active site (lower left—see text for details). For simplicity, the N-terminal unique domain and regulatory C-terminal tail are shown only for the closed/inactive state.