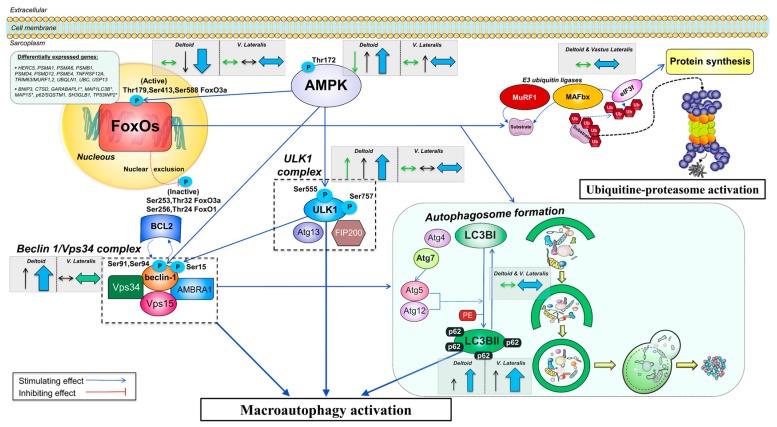
Figure 6.
Schematic representation of the regulation of the autophagy-lysosome system in response to severe energy deficit and its modulation by exercise. An upregulation of numerous genes involved in the ubiquitin-proteasome and the autophagy-lysosome degradation pathways is seen after the CRE phase, primarily in the vastus lateralis. Elevated levels of cortisol with low insulin, testosterone, and leptin levels did not prevent increased inhibitory phosphorylation of FoxO1 and FoxO3a in the deltoid muscles, which was blunted by prolonged low-intensity exercise in the vastus lateralis, despite inactivation of the PI3K/Akt/mTOR pathway [52]. FoxOs orchestrate the activation of the ubiquitin-proteasome and autophagy-lysosomal degradation pathways. Upon FoxOs unchanged or reduced activation, the levels of E3 ligases MuRF1 and MAFbx are not augmented, limiting the ubiquitin-mediated tagging of proteins for proteasomal degradation. The central role in the antagonistic regulation of catabolic and anabolic signals driven by eIF3f is not altered as a consequence of its MAFbx-dependent degradation. AMPK can activate FoxO3a via phosphorylation or independently stimulate degradation via the autophagy-lysosomal pathway. A severe energy deficit induces autophagy activation via higher Thr172 AMPKα in all muscles, although the augmented fractional phosphorylation at Thr172 solely present in the deltoid muscles indicates inactivation by a large exercise volume in the lower extremities. AMPKα directly increases activation of ULK1 through phosphorylation at Ser555, an effect abrogated in the vastus lateralis by high-volume exercise. AMPK and ULK1 propagate downstream signaling by altering the regulation of the Beclin 1/Vps34 complex, whose fine integration and functioning is required for complete autophagosome formation. An increased phosphorylated Beclin 1 at Ser15 can be sufficient to regulate the levels of the entire complex, and likely the augmented phosphorylation present only in the deltoid muscles elicits stimulation of autophagy induction, which was not present in the vastus lateralis. No sign of increased LC3BII or conversion of LC3BI to LC3BII is suggestive of an overall unchanged rate of autophagosome formation. The enhanced accumulation of the sequestosome 1 (p62/SQSTM1) in all muscles but preferentially in the vastus lateralis, is supportive of an inhibition of autophagic flux modulated by the volume of exercise performed by the skeletal muscles, as p62/SQSTM1 is an acceptor of ubiquitinated substrates whose expression should be lowered following lysosomal degradation. The arrows included inside dashed grey boxes illustrate the overall protein expression changes in this investigation split in the deltoid muscles (mean of both arms) and vastus lateralis and are shown beside the specific markers. Thin arrows in green (total form) and black (phosphorylated form) depict the overall direction of the outcomes (increase/decrease) for the particular muscle group. Thick arrows in blue depict the overall effect on stimulation/inhibition of the ubiquitin-proteasome or autophagy-lysosomal degradation pathways. The size of the arrow is representative of the magnitude of the change. Activatory/inhibitory actions are represented by blue/red connecting lines (dashed if changes in location are present). Differentially expressed genes refer to an upregulation unless an asterisk (*) is placed on the gene name (downregulation). Abbreviations not defined in the text: AMBRA1, activating molecule in BECN1 regulated autophagy protein 1; Atg4/5/7/12/13, autophagy-related 4/5/7/12/13; BCL2, B-cell lymphoma 2; FIP200, focal adhesion kinase family-interacting protein of 200 kDa; Vps15, vacuole protein sortin 15.