Abstract
Background:
Increases in ambient temperature have recently been associated with increased emergency department visits and hospital admissions for acute renal failure. However, potential biological mechanisms through which short-term ambient temperature affects kidney function are not known.
Methods:
We used multiple regression models to evaluate the association between 1- and 3-day average, ambient temperature levels and two biomarkers of kidney injury (neutrophil gelatinase-associated lipocalin (NGAL) and adiponectin), among 3377 individuals over 57 years of age enrolled in the National Social Life, Health, and Aging Project. Ambient temperature was estimated on a 6-km grid covering the conterminous United States using ambient temperature measurements obtained from the National Climatic Data Center (NCDC). NGAL and adiponectin levels were measured from whole blood collected for each participant. All health effect models were adjusted for a number of demographics, socioeconomic, health behavior, medical history variables, with non-linear exposure-response relationships examined using natural cubic splines.
Results:
The relationship between 1- and 3-day average temperature and both NGAL and adiponectin levels was significant and non-linear, with largely null associations below 10 °C, and positive association for temperatures > 10 °C. In fully adjusted, linear multiple regression models restricted to > 10 °C, NGAL and adiponectin levels increased by 1.89% (95% CI: 0.77, 3.91) and 2.51% (95% CI: 1.34, 3.69), respectively, for a 1 °C increase in daily average temperature. Additionally, every 1 °C increase in temperature over 10 °C was associated with a 1.83% increased odds of having plasma NGAL levels consistent with acute kidney injury (> 150 μg/L).
Conclusions:
In a cohort of older men and women in the United States, our study is the first to observe that short-term ambient temperature exposures were significantly associated with biomarkers of kidney injury. These associations suggest that ambient temperature exposures could be an important risk factor for renal pathology.
Keywords: Ambient temperature, Elderly, Adiponectin, NGAL, Kidney injury
1. Introduction
Variations in ambient temperature have been associated with significant increases in morbidity and mortality, particularly among the elderly (Song et al., 2017). Numerous prior studies have identified high ambient temperature to be associated with increased risk of acute myocardial infarction, congestive heart failure, stroke, and respiratory disease, while conversely, low temperatures have been shown to increase cardiovascular and cerebrovascular risk (Ebi et al., 2004; Schwartz and Patz, 2004; Bayentin et al., 2010; Koken et al., 2003; Wang et al., 2009; Green et al., 2009; Dawson et al., 2008; Liang et al., 2008; Panagiotakos et al., 2004; Ohshige et al., 2006; Chang et al., 2004). The effect of extreme temperature on renal disease has been explored extensively, particularly with regard to extreme heat events and hospital admissions for kidney disorders (Basu et al., 2012; Bobb et al., 2014; Fletcher et al., 2012; Gronlund et al., 2014; Lin et al., 2013; Semenza et al., 1999). However, no prior studies have investigated whether measurable, subclinical renal injury occurs at elevated, but non-extreme temperatures. As temperature changes can have significant impacts on hemodynamics and blood pressure, it is possible that less extreme temperature shifts in susceptible individuals also contribute to subclinical and incremental renal injury.
Renal disease is the 9th most common cause of death in the United States. A major contributor to renal disease is chronic kidney disease (CKD), which is present in an estimated 14% of the US population, with its prevalence substantially increased in the last quarter of the 20th century both in the US and worldwide (Kidney Disease Statistics for the United States, 2018; Hsu and Powe, 2017). While some of this increase is explainable by increases in the prevalence of known risk factors such as high blood pressure and diabetes mellitus, increases in extreme temperature events have also emerged as a potential cause in certain populations. Because CKD is chronic, progressive, and asymptomatic in its early stages, detrimental renal effects of repeated exposure to higher temperatures could be difficult to detect using standard, clinical measures, such as serum creatinine (Hsu and Powe, 2017).
Serum creatinine is often used to evaluate renal function and the presence of CKD. However, it has a number of specific weaknesses which diminish its utility in the evaluation of sub-clinical kidney injury. For example, serum creatinine is an insensitive indicator of early, acute changes in kidney function and may not appreciably change until approximately half of kidney function has already been lost (Wheeler et al., 2008). For this reason, biomarkers of kidney injury (as opposed to clinical markers of kidney function) are better able to assess for sub-acute/subclinical renal insults. Specifically, neutrophil gelatinase-associated lipocalin (NGAL) and adiponectin have been previously established as important biomarkers of kidney injury (Haase et al., 2009). NGAL has been shown in animal models to be one of the most strongly upregulated genes, and among the earliest and most robustly induced proteins in the kidney after ischemic or nephrotoxic kidney insult (Supavekin et al., 2003; Devarajan et al., 2003; Mishra et al., 2003, 2004a, 2004b; Mori et al., 2005; Schmidt-Ott et al., 2006, 2007). Importantly, it has also been shown to accurately identify subacute kidney injury when the renal insult is too small to causes changes in serum creatinine, yet large enough to increase the risk of adverse clinical outcomes (Haase et al., 2011). In prior studies, absolute NGAL levels over 150 μg/L were strongly predictive of acute kidney injury (73% sensitivity, 81% specificity), while increases in NGAL from baseline of 200–400 percent have been documented in young agricultural workers exposed to extreme temperatures (Cruz et al., 2010; Wesseling et al., 2016a). Similarly, adiponectin, a protein secreted by adipose tissue that modulates glucose and lipid metabolism, has been recently identified as having important roles in both acute and chronic kidney injury (Zoccali CM, 2007). While the exact physiological mechanism by which adiponectin impacts the kidney during an acute renal insult is not well understood, animal models suggest that ischemic injury to the kidney may upregulate adiponectin transcription, leading to invasion of proinflammatory cells and subsequent renal tubulointerstitial fibrosis (Oh and Rabb, 2013).
These markers may be of particular importance in the study of older individuals across all ambient temperature ranges. Numerous studies have shown that the elderly are particularly susceptible to the deleterious effects of extreme temperatures, and especially extreme high temperatures (Bouchama, 2003; Impact of heat waves on m, 2004; Curriero et al., 2002; Greenberg et al., 1983; Grize et al., 2005). Research has increasingly shown biomarkers of kidney injury to increase with extreme heat in young adult outdoor workers, such as sugar cane harvesters in Central America. Whether such impacts also occur at non-extreme variations in temperature are not known, particularly in the elderly who may be particularly vulnerable given their diminished physiological reserve due to age-related changes in cardiovascular health and thermoregulatory capacity (Wesseling et al., 2016a, 2016b; Hess et al., 2009; Mercer, 2003; Smolander, 2002; Glaser et al., 2016).
To assess the potential impact of temperature on kidney function, we examine associations between short-term ambient temperature and serum NGAL and adiponectin levels in a nationally representative cohort of older Americans.
2. Methods
2.1. Population
We used demographic, health, and other data from a nationally representative probability sample of Americans participating in the National Social Life, Health, and Aging Project (NSHAP). NSHAP is a national area probability sample of community residing, older (57+ year) adults, selected from households identified in the Health and Retirement Study (a national, multi-stage area probability sample with a target population of all U.S. adults) in 2004 (Waite et al., 2014a, 2014b; Honda et al., 2017). Participants included 3377 participants who were interviewed and provided biosamples in 2011 and 2012. The survey over-sampled African-Americans, Latinos, men and individuals between 75 and 84 years (Waite et al., 2014a). Response rates were high, with 74% of individuals opting to participate. Participants underwent interviews to obtain demographic, social (social networks, social support, marital history and intimate partnerships and sexuality), and health (self-reported health, physical function and morbidity) data, including medical history and a comprehensive list of current medications. At the time of the interviews, biomeasure data on anthropometrics (height, weight, waist circumference), cardiovascular health (blood pressure, pulse), and biological samples (blood samples) were also collected (Waite et al., 2014a, 2014b).
2.2. Outcome assessment
NGAL and adiponectin measurements were collected from whole blood samples following standard protocols in participants’ residences (O’Doherty et al., 2014). Trained interviewers collected five drops (250 μL) of whole blood in Microtainers coated with dipotassium ethylenediaminetetraacetic acid to prevent clotting. Biosamples were immediately cooled and transported in controlled temperature packages to the University of Chicago Flow Cytometry Facility. The unclotted blood was centrifuged and the plasma was extracted and frozen at the University of Chicago, Flow Cytometry Facility. Multiplex magnetic-bead antibody kits with a Luminex reader was used to analyze samples and estimate analyte levels (O’Doherty et al., 2014). Supplementary Figs. 1 and 2 show boxplots of NGAL and adiponectin levels by month of blood collection.
2.3. Exposure assessment
Ambient temperature estimates were based upon measurements obtained from the National Climatic Data Center (NCDC). Consistent with previous literature, splines were used to estimate temperatures for a 6 km grid of the conterminous United States (Luo et al., 1998). The temperature estimates derived from these models were used to calculate 1- and 3-day moving average ambient temperature exposure estimates. 1- and 3-day moving averages were selected as the exposure measure given their association in previous studies with increased kidney biomarker levels within 24–72 h of exposure (Haase et al., 2009; Wheeler and Wong, 2008). NSHAP participants were assigned exposure estimates based on the grid point closest to their permanent addresses and the day of health of measurement (mean distance 2.23 km) (Yanosky et al., 2014).
We estimated exposures to short-term ambient fine particles (PM2.5) outside each participant’s home using spatio-temporal models as described in Yanosky et al. (2014). NSHAP participants were matched to the grid point closest to his/her self-reported residential address (mean distance 2.23 km). Nitrogen dioxide (NO2) exposures were estimated using measurements from the nearest AQS monitor within 80 km of NSHAP participants’ residential addresses, with the median distance to a monitor being 11.4 km (IQR 32.2).
2.4. Covariates
We controlled for a number of potential confounders in our models, including: anthropometric, demographic, socio-economic, behavioral, medical, and other residential measures. We selected these covariates based on their previously observed associations with either kidney function or temperature. Anthropometric measurements included body mass index (BMI, kg/m2) (Voss et al., 2013; Eriksen and Ingebretsen, 2006), demographic factors included age, sex, and race/ethnicity categorized as White, Black, Hispanic or other (Melamed et al., 2009). Socioeconomic status (SES) was assessed on an individual level using self-reported educational attainment, and on a neighborhood level using median household income and percent of households below the federal poverty line as reported in the 2000 US Census (Patzer and McClellan, 2012). Behavioral factors included smoking status (current, historical, or none) (Shankar et al., 2006), self-reported frequency of physical activity, and alcohol use (ever versus none) (Mora-Rodriguez et al., 2016; Das Kumar SV, 2008). Medical variables included self-reported history of diabetes and hypertension (Yang et al., 2016; Woodhouse et al., 1993). Covariates describing season, region of residence, and urbanicity were included as categorical variables. Specifically, season was classified as Spring (March–May), Summer (June–August), Fall (September–November), and Winter (December–February); region of residence as five categories: North Atlantic, South, Great Lakes region, Plains States, Pacific; and urbanicity as six categories (12 largest Standard Metropolitan Statistical Areas (SMSAs), 13–100 largest SMSAs, 12 largest suburbs, 13–100 largest suburbs, Other urban, and Other rural). (Joyce Fan et al., 2007).
2.5. Statistical analysis
As prior literature has shown effects of temperature to be significantly non-linear, we used natural cubic splines to examine non-linear associations (Bayentin et al., 2010; Liang et al., 2008; Lee, 2003). We subsequently investigated linear associations above the thresholds identified in the natural cubic splines between 1- and 3-day ambient average temperature and log-transformed NGAL and adiponectin levels in base and fully adjusted multiple linear regression models. Base linear models controlled for age, sex, and BMI (Model 1); intermediate models additionally controlled for season, region, urbanicity, current/historical tobacco use, physical activity, alcohol use (Model 2); and fully adjusted models additionally for neighborhood characteristics (race, education, median household income, percent poverty) and participant disease status (diabetes, hypertension). The inverse logarithm of coefficients was calculated and results are presented as percent increases in biomarkers. Additionally, we used logistic models to examine the associations between ambient temperature and odds of having NGAL levels consistent with acute kidney injury (defined as > 150 μg/L). Effect modification for linear and logistic models was investigated by inclusion of a multiplicative interaction term for sex, age (as above or below the median), BMI (as above or below the median), smoking status, education, history of hypertension and diabetes, region, and race/ethnicity. All models were weighted to account for non-response and oversampling of certain populations.
Data completeness for all variables of interest was high, with missingness for NGAL and adiponectin at 20.2% and 20.5%, BMI missing 5.5%, and all other covariates with < 1% missingness. Multiple imputation with chained equations was used to handle all covariate and outcome variable missingness by creating ten datasets with complete data and pooling estimates across datasets using standard procedures (White et al., 2011). In Supplement Table 3, we have provided a comparison of covariates and exposures of interest for those with and without outcome measurements. For almost all covariates measured, there were no statistically significant differences in covariates between those with and without missing outcomes. The only exception was race/ethnicity, where small, significant variations were seen in the distribution of white and black participants with missingness (approximately 5% difference between missing and non-missing groups in each ethnic group, respectively). As all covariates in Supplement Table 3 were included in our multiple imputation models, however, differences based upon this observed covariate should not have biased the imputation effect estimates, assuming data are Missing at Random (MAR). (Cummings, 2013; Sinharay et al., 2001).
We conducted sensitivity analyses to ensure our findings are robust to various model specifications. First, variables that are potentially on the causal pathway between ambient temperature and kidney disease (i.e., BMI, history of diabetes and hypertension) were excluded from models. Second, complete case analyses were undertaken to examine the potential effects of the imputation methods. Third, we controlled for ambient PM2.5 and NO2 as potential confounders. Analyses were completed using SAS Version 9.4 (SAS INC, Cary, NC).
3. Results
3.1. Participant characteristics
Table 1 summarizes the characteristics of the cohort. The mean age of the participants was 72.4 years. The majority of participants were female (54.5%), white (71.5%), and college educated (56.2%). Participants tended to be overweight, with a mean BMI of 29.3 kg/m2. In addition, 82.9% of the participants reported a history of hypertension and 24.4% of diabetes. The median NGAL and adiponectin levels equaled 9.5 μg/L (± 12.8) and 518.1 μg/L (± 574.5), respectively, while the mean daily temperatures equaled 14.1 °C (± 8.9).
Table 1.
Cohort characteristics.
| Total Observations (n = 3377) | |
|---|---|
| Demographics | |
| Age (mean, SD) | 72.4 ± 8.1 |
| Male (n, %) | 1538 (45.5%) |
| Race/Ethnicity | |
| White (n, %) | 2415 (71.5%) |
| Black (n, %) | 517 (15.3%) |
| Hispanic (n, %) | 367 (10.9%) |
| Other (n, %) | 78 (2.3%) |
| Health Status | |
| BMI kg/m2 (mean, SD) | 29.3 ± 6.2 |
| History of hypertension (n, %) | 2799 (82.9%) |
| History of diabetes (n, %) | 824 (24.4%) |
| NGAL μg/L (median, IQR) | 9.5 ± 12.8 |
| Adiponectin μg/L (median, IQR) | 518.1 ± 574.5 |
| Behavioral Variables | |
| Current tobacco use | |
| Yes (n, %) | 450 (13.3%) |
| No (n, %) | 2927 (86.7%) |
| SES | |
| Education | |
| < College degree (n, %) | 1478 (43.8%) |
| College degree or greater (n, %) | 1899 (56.2%) |
| Median household income (mean, SD) | 56,438 ± 27, 262 |
| Percent census track below poverty line (mean, SD) | 14.4% ± 11.9% |
| Temperature (mean, SD) | |
| Daily average temperature (°C) | 14.1 ± 8.9 |
| 3-day moving average (° C) | 14.1 ± 8.8 |
3.2. NGAL and ambient temperature
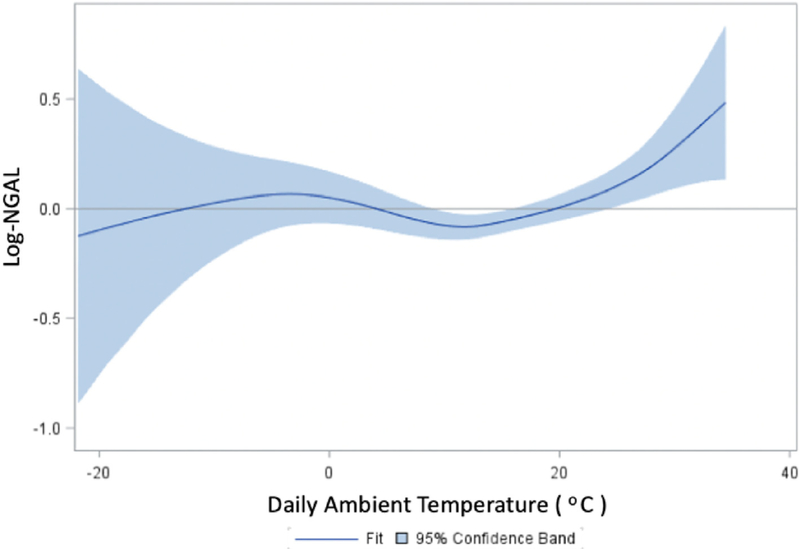
Table 2 presents the associations between NGAL levels and ambient temperature. In base models, a 1 °C increase in daily average temperature was associated with a 1.2% increase in NGAL levels (95% CI: 0.56, 1.48). After adjustment for anthropometric, demographic, socioeconomic, behavioral, medical, and neighborhood measures, the temperature-NGAL association was attenuated and no longer statistically significant (0.51%, 95% CI: −0.12, 1.14). When the association was modeled using natural cubic splines, we found a significant non-linear relationship between ambient temperature and NGAL, with largely null associations below 10 °C, and positive associations for temperatures greater than 10 °C (Fig. 1). Correspondingly, in fully adjusted linear multiple regression models restricted to temperatures greater than 10 °C, we found a 1 °C increase in daily average temperature to be associated with 1.89% (95% CI: 0.77, 3.91) higher NGAL levels. Associations were similar for 3-day moving average temperatures greater than 10 °C in fully adjusted models (1.77%, 95% CI: 0.61, 2.93). Additionally, in logistic models, every 1 °C increase in temperature over 10 °C was associated with a 1.83% increased odds of having plasma NGAL levels > 150 μg/L.
Table 2.
Associations between NGAL levels and ambient temperatures.
| Temperature Measure | Model 1 (base) | Model 2 (intermediate) | Model 3 (full) |
|---|---|---|---|
| Daily temp | 1.02 (0.56, 1.48) | 0.37 (−0.24, 0.99) | 0.51 (−0.12, 1.14) |
| Daily > 10 °C | 2.01 (0.99, 3.10) | 1.62 (0.50, 2.73) | 1.89 (0.77, 3.01) |
| 3 day > 10 °C | 1.94 (0.84, 3.03) | 1.48 (0.34, 2.63) | 1.77 (0.61, 2.93) |
Model 1: temperature, age, sex, BMI.
Model 2: Model 1 + season, region, urbanicity, current/historical tobacco use, physical activity, alcohol use.
Model 3: Model 2 + neighborhood characteristics (race, education, median household income, percent poverty), diabetes, hypertension.
Fig. 1.
Dose response curve for daily temperature and NGAL.
3.3. Adiponectin and ambient temperature
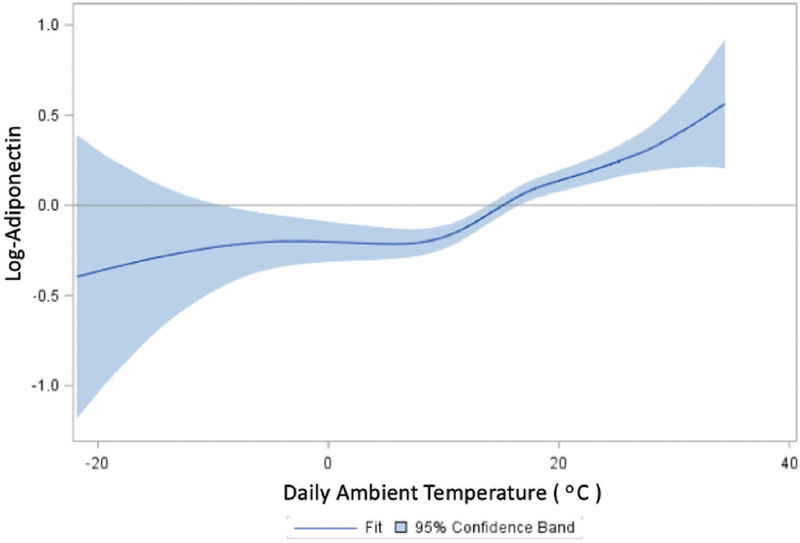
Table 3 presents the associations between NGAL levels and ambient temperature. In base models, a 1 °C increase in daily average temperature was associated with a 1.2% increase in NGAL levels (95% CI: 0.56, 1.48). After adjustment for anthropometric, demographic, socioeconomic, behavioral, medical, and neighborhood measures, the temperature-NGAL association was attenuated and no longer statistically significant (0.51%, 95% CI: −0.12, 1.14). When the association was modeled using natural cubic splines, we found a significant non-linear relationship between ambient temperature and NGAL, with largely null associations below 10 °C, and positive associations for temperatures greater than 10 °C (Fig. 1). Correspondingly, in fully adjusted linear multiple regression models restricted to temperatures greater than 10 °C, we found a 1 °C increase in daily average temperature to be associated with 1.89% (95% CI: 0.77, 3.91) higher NGAL levels. Associations were similar for 3-day moving average temperatures greater than 10 °C in fully adjusted models (1.77%, 95% CI: 0.61, 2.93). Additionally, in logistic models, every 1 °C increase in temperature over 10 °C was associated with a 1.83% increased odds of having plasma NGAL levels > 150 μg/L presents the associations between adiponectin levels and ambient temperature. In the full cohort, a 1 °C increase in daily average temperature was associated with a 1.98% (95% CI: 1.28, 2.68) increase in adiponectin levels. As with NGAL, exposure-response curves showed a highly non-linear association, with strong, positive associations between adiponectin and temperatures > 10 °C, while associations for lower temperatures were largely null (Fig. 2). In fully adjusted multiple regression models restricted to > 10 °C, a one-degree increase in average temperature was associated with between 2.51% (95% CI: 1.34, 3.69) and 2.62% (95% CI: 1.38, 3.87) higher levels of adiponectin for one- and three-day moving averages, respectively.
Table 3.
Associations between adiponectin levels and ambient temperatures.
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Daily temp | 1.90 (1.38, 2.32) | 1.79 (1.09, 2.48) | 1.98 (1.28, 2.68) |
| Daily > 10 °C | 2.26 (1.21, 3.30) | 2.23 (1.06, 3.39) | 2.51 (1.34, 3.69) |
| 3 day > 10 °C | 2.29 (1.17, 3.40) | 2.30 (1.06, 3.55) | 2.62 (1.38, 3.87) |
Model 1: Temperature age sex BMI.
Model 2: Model 1 + Season, region, urbanicity, current/historical tobacco use, physical activity, alcohol use.
Model 3: Model 2 + neighborhood characteristics (race, education, median household income, percent poverty), diabetes, hypertension.
Fig. 2.
Dose response curve for daily temperature and adiponectin.
3.4. Effect modification and sensitivity analyses
Supplementary Table 1 presents the results of our effect modification analyses. We found a suggestion the magnitude of associations between temperature and NGAL is higher in older individuals (as defined as being older than 70 years, the median age, PInteract = 0.090). No other covariates were found to modify the NGAL-temperature association. Sensitivity analyses using complete cases and excluding potential causal intermediates (Supplementary Table 2) and controlling for ambient particulate matter (PM2.5) did not meaningfully alter our findings.
4. Discussion
Our study is the first to investigate associations between biomarkers of kidney injury and ambient 1- and 3-day average temperature exposures in older Americans. We observed that associations between temperature and both NGAL and adiponectin were non-linear, with null associations below approximately 10 °C and significantly positive associations at higher ambient temperatures. Importantly, our findings were clinically relevant, as we showed the odds of having NGAL levels above 150 μg/L, the clinical threshold that is highly predictive of acute kidney injury, to increase with ambient temperature (Cruz et al., 2010). Consistent with this, our finding of effect modification by age may indicate that older adults are particularly vulnerable to the effects of increased temperature. Overall, the associations we observe suggest that ambient temperature may be an important factor in renal pathology, particularly in the elderly, who are known to have diminished physiological reserve due to age-related changes in cardiovascular health and thermoregulatory capacity (Hess et al., 2009; Mercer, 2003; Smolander, 2002).
Although no other studies have examined the association of kidney biomarker levels and ambient temperature, our findings are consistent with the limited prior literature exploring the associations between ambient temperature and renal disease, as assessed using hospital and emergency department admissions data (Basu et al., 2012; Bobb et al., 2014; Fletcher et al., 2012; Gronlund et al., 2014; Lin et al., 2013). In a cohort in California, Basu et al. (2012) identified that every 10 °F increase in daily ambient temperature was associated with 15.9% excess risk (95% CI: 12.7 to 19.3) of emergency department admission for acute renal failure. Similarly, a 2014 study of Medicare enrollees from across the United States found that risks of hospitalization for renal failure during heat wave periods increased by 14% (RR 1.14, 95% CI: 1.06, 1.23), while a similar study of inpatient Medicare records by Gronlund et al. (2014) found extreme heat days to be associated with 15% increased risk of renal failure admission (95% CI: 9%, 21%) (Bobb et al., 2014; Gronlund et al., 2014). Fletcher et al. found similar associations in a large (> 140,000) cohort of New York State admissions data, with a 5 °F increase in 1-day lagged temperature was associated with 9% increased odds of hospital admission for acute renal failure (OR 1.09, 95% CI: 1.07, 1.12) (Fletcher et al., 2012). Similar to the non-linear associations we observed in our study, in a Taiwanese population, Lin et al. (2013) observed a J-shaped association between ambient temperature and acute renal hospital admissions, with the lowest risk for renal admissions observed at an ambient temperature of 25 °C, with risks increasing monotonically as temperatures deviated upwards (Lin et al., 2013).
These studies of admissions outcomes treat kidney function as a binary outcome, where acute decompensation resulting in seeking medical care is the result of some pathophysiologic threshold beyond which acute disease manifests. Particularly in the case of kidney disease, this may not be true. Take, for example, the emergence of Mesoamerican Nephropathy. This is a postulated disorder to explain the emergence of a chronic, progressive nephropathy in Mesoamerica which has resulted in nearly 20,000 deaths over the last two decades and appears to be increasing in frequency, likely due to increases in global temperature, and in particular, the impact that climate change has on the number and severity of extreme temperature days in Central America (Trabanino et al., 2002; vWdJBCrowe et al., 2015; Ramirez-Rubio et al., 2013). One proposed mechanism for Mesoamerican Nephropathy, supported by animal models, is repeated heat insults resulting in sub-clinical and progressive tubulointerstitial fibrosis (Roncal-Jimenez et al., 2016). Importantly, this sub-clinical and progressive damage occurred without the dramatic changes in intravascular volume and blood pressure classically associated with dehydration-induced acute kidney failure. Thus, it is possible that the renal effects of higher ambient temperature have both acute (e.g. resulting in hospital and emergency department admissions) as well as sub-acute (repeated, cumulative heat-related insults leading progressively to tubular fibrosis) components, the latter of which is largely unexplored in the current literature. This is consistent with our findings, in which increases in temperature were associated with increased odds of participants having clinically important high NGAL levels. Supporting this, one prior study of Nicaraguan laborers identified that work in extreme heat environments was associated with 4-fold increases in NGAL, while a similar study in a Brazilian cohort found that a day of labor in extreme heat was associated with increases in markers of acute kidney injury (including: creatinine phosphokinase, malondialdehyde levels, increased white cell counts, decreased urinary and serum sodium, decreased fractional excretion of sodium, and increased urine concentration) (Wesseling et al., 2016a; Santos Ubiratan et al., 2015). These prior studies are limited, in that they were conducted in young, non-US populations, and did not explicitly associate variations in ambient temperature to the findings of abnormal kidney function, thus limiting their applicability to our study.
Our study has a number of important limitations. For example, the analysis is cross-sectional, and thus temporality is unknown. Also, we use ambient temperature as our exposure measure, which does not capture the temperatures experienced by our study cohort in indoor and other environments. These limitations are counterbalanced by a number of significant strengths. First, we utilize the NSHAP cohort, which makes it the first to investigate these associations in a nationally representative sample of older adults. Second, the availability of high-quality data on multiple potential confounders decreases the likelihood that the observed effects are entirely due to uncontrolled confounding. Third, our findings are consistent with previous literature examining the effects of temperature on renal pathology. Last, associations were robust to a number of sensitivity analyses.
5. Conclusions
Short-term ambient temperatures were found to have a non-linear relationship with biomarkers of kidney disease, such that temperatures over 10 °C were significantly associated with increases in two important biomarkers of renal injury (NGAL and adiponectin) in an older, US population. These associations suggest that acute increases in ambient temperature may be an important and increasing risk factor for sub-acute kidney injury in older US adults.
Supplementary Material
Acknowledgements
We thank Dr. Jeffrey Yanosky from the Pennsylvania State University for providing daily PM2.5 grid data.
Funding
This work was supported by NIEHS grant 1R01ES022657-01A1, with health and other covariate data obtained through NIH R01-AG021487, R37-AG030481, R01-AG033903, and R01-ES019168.
Abbreviations:
- NGAL
neutrophil gelatinase-associated lipocalin
Footnotes
Ethics approval and consent to participate
This study was approved by the institutional review board (IRB) of Northeastern University and all participants provided written informed consent: IRB# 13-07-18.
Declaration of competing interest
The authors declare that there is no duality of interest associated with this manuscript.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2019.108790.
References
- Basu R, Pearson D, Malig B, Broadwin R, Green R, 2012. The effect of high ambient temperature on emergency room visits. Epidemiology 23 (6), 813–820. [DOI] [PubMed] [Google Scholar]
- Bayentin LEAS, Ouarda TB, Gosselin P, Doyon B, Chebana F, 2010. Spatial variability of climate effects on ischemic heart disease hospitalization rates for the period 1989–2006 in Quebec, Canada. Int. J. Health Geogr 9 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Obermeyer Z, Wang Y, Dominici F, 2014. Cause-specific risk of hospital admission related to extreme heat in older adults. J. Am. Med. Assoc 312 (24), 2659–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchama A, 2003. European heat wave. Intensive Care Med. 30, 1–3. [DOI] [PubMed] [Google Scholar]
- Chang CLSM, Marmot M, Poulter N, 2004. Lower ambient temperature was associated with an increased risk of hospitalization for stroke and acute myocardial infarction in young women. J. Clin. Epidemiol 57, 749–757. [DOI] [PubMed] [Google Scholar]
- Cruz DN, de Cal M, Garzotto F, et al. , 2010. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med. 36 (3), 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings P, 2013. Missing data and multiple imputation. JAMA Pediatr. 167 (7), 656–661. [DOI] [PubMed] [Google Scholar]
- Curriero FCHK, Samet JM, Zeger SL, Strug L, Patz JA, 2002. Temperature and mortality in 11 cities of the eastern United States. Am. J. Epidemiol 155, 80–87. [DOI] [PubMed] [Google Scholar]
- Kumar S Das, V DM, 2008. Indian J Clin Biochem Alcohol induced effects on kidney. Indian J. Clin. Biochem 23 (1), 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson JWC, Wright F, Bryden C, Aslanyan S, Lees K, et al. , 2008. Associations between meteorological variables and acute stroke hospital admissions in the west of Scotland. Acta Neurol. Scandanavia 117, 85–89. [DOI] [PubMed] [Google Scholar]
- Devarajan PMJ, Supavekin S, Patterson LT, Potter SS, 2003. Gene expression in early ischemic renal injury: clues towards pathogenesis, biomarker discovery, and novel therapeutics. Mol. Genet. Metab 80, 365–376. [DOI] [PubMed] [Google Scholar]
- Ebi KLEK, Lau E, Kelsh M, Barnston A, 2004. Weather changes associated with hospitalizations for cardiovascular diseases and stroke in California, 1983–1998. Int. J. Biometeorol 49, 48–58. [DOI] [PubMed] [Google Scholar]
- Eriksen BO, Ingebretsen OC, 2006. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 69 (2), 375–382. [DOI] [PubMed] [Google Scholar]
- Fletcher BA, Lin S, Fitzgerald EF, Hwang S-A, 2012. Association of summer temperatures with hospital admissions for renal diseases in New York state: a case-crossover study. Am. J. Epidemiol 175 (9), 907–916. [DOI] [PubMed] [Google Scholar]
- Glaser J, Lemery J, Rajagopalan B, et al. , 2016. Climate change and the emergent epidemic of CKD from heat stress in rural communities: the case for heat stress nephropathy. Clin. J. Am. Soc. Nephrol 11 (8), 1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RSBR, Malig B, Broadwin R, Kim JJ, Ostro B, 2009. The effect of temperature on hospital admissions in nine California counties. Int. J. Public Health 55, 113–121. [DOI] [PubMed] [Google Scholar]
- Greenberg JHBJ, Reed CM, Gustafson TL, Beauchamp RA, 1983. The epidemiology of heat-related deaths, Texas—1950, 1970–79, and 1980. Am. J. Public Health 73, 805–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grize LHA, Thommen O, Schindler C, Braun-Fahrlander C, 2005. Heat wave 2003 and mortality in Switzerland. Swiss Med. Wkly 135, 200–205. [DOI] [PubMed] [Google Scholar]
- Gronlund CJ, Zanobetti A, Schwartz JD, Wellenius GA, O’Neill MS, 2014. Heat, heat waves, and hospital admissions among the elderly in the United States, 1992–2006. Environ. Health Perspect 122 (11), 1187–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A, 2009. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am. J. Kidney Dis 54 (6), 1012–1024. [DOI] [PubMed] [Google Scholar]
- Haase M, Devarajan P, Haase-Fielitz A, et al. , 2011. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury. A Multicenter Pooled Analysis of Prospective Studies. J. Am. Coll. Cardiol 57 (17), 1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess KL, Wilson TE, Sauder CL, Gao Z, Ray CA, Monahan KD, 2009. Aging affects the cardiovascular responses to cold stress in humans. J. Appl. Physiol 107 (4), 1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T, Pun VC, Manjourides J, Suh H, 2017. Anemia prevalence and hemoglobin levels are associated with long-term exposure to air pollution in an older population. Environ. Int 101, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu RK, Powe NR, 2017. Recent trends in the prevalence of chronic kidney disease: not the same old song. 26 (3), 187–196. [DOI] [PubMed] [Google Scholar]
- Impact of Heat Waves on Mortality—Rome, Italy, Jun-Aug 2003. CDC. [PubMed] [Google Scholar]
- Joyce Fan Z, Lackland DT, Lipsitz SR, et al. , 2007. Geographical patterns of end-stage renal disease incidence and risk factors in rural and urban areas of South Carolina. Health Place 13 (1), 179–187. [DOI] [PubMed] [Google Scholar]
- Kidney Disease Statistics for the United States, 2018. National Institute of Health. National Institute of Diabetes and Digestive and Kidney Disease. https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease.
- Koken PJPW, Ye F, Elixhauser A, Olsen LM, Portier CJ, 2003. Temperature, air pollution, and hospitalization for cardiovascular diseases among elderly people in Denver. Environ. Health Perspect 111, 1312–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TCM, 2003. Smoothing parameter selection for smoothing splines: a simulation study. Comput. Stat. Data Anal 42 (1), 139–148. [Google Scholar]
- Liang WMLW, Chou SY, Kuo HW, 2008. Ambient temperature and emergency room admissions for acute coronary syndrome in Taiwan. Int. J. Biometeorol (52), 223–229. [DOI] [PubMed] [Google Scholar]
- Lin Y-K, Wang Y-C, Ho T-J, Lu C, 2013. Temperature effects on hospital admissions for kidney morbidity in Taiwan. Sci. Total Environ 443, 812–820. [DOI] [PubMed] [Google Scholar]
- Luo Z, Wahba G, Johnson DR, 1998. Spatial–temporal analysis of temperature using smoothing spline ANOVA. J. Clim 11 (1), 18–28. [Google Scholar]
- Melamed ML, Astor B, Michos ED, Hostetter TH, Powe NR, Muntner P, 2009. 25-Hydroxyvitamin D levels, race, and the progression of kidney disease. J. Am. Soc. Nephrol 20 (12), 2631–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JB, 2003. Cold—an underrated risk factor for health. Environ. Res 92 (1), 8–13. [DOI] [PubMed] [Google Scholar]
- Mishra JMQ, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. , 2003. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol 4, 2534–2543. [DOI] [PubMed] [Google Scholar]
- Mishra JMK, Ma Q, Kelly C, Barasch J, Devarajan P, 2004a. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am. J. Nephrol 24, 307–315. [DOI] [PubMed] [Google Scholar]
- Mishra JMK, Ma Q, Kelly C, Yang J, Mitsnefes M, et al. , 2004b. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J. Am. Soc. Nephrol 15, 3073–3082. [DOI] [PubMed] [Google Scholar]
- Mora-Rodriguez R, Ortega JF, Fernandez-Elias VE, et al. , 2016. Influence of physical activity and ambient temperature on hydration: the European hydration research study (EHRS). Nutrients 8 (5), 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori KLH, Rapoport D, Drexler IR, Foster K, Yang J, et al. , 2005. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J. Clin. Investig 115, 610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Rabb H, 2013. Adiponectin: an enlarging role in acute kidney injury. Kidney Int. 83 (4), 546–548. [DOI] [PubMed] [Google Scholar]
- Ohshige KHY, Tochikubo O, Sugiyama M, 2006. Influence of weather on emergency transport events coded as stroke: population-based study in Japan. Int. J. Biometeorol 50, 305–311. [DOI] [PubMed] [Google Scholar]
- O’Doherty K, Jaszczak A, Hoffmann JN, et al. , 2014. Survey field methods for Expanded biospecimen and biomeasure collection in NSHAP wave 2. J. Gerontol.: Ser. B 69 (Suppl_2), S27–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotakos DBCC, Pitsavos C, Nastos P, Anadiotis A, Tentolouris C, et al. , 2004. Climatological variations in daily hospital admissions for acute coronary syndromes. Int. J. Cardiol 94, 229–233. [DOI] [PubMed] [Google Scholar]
- Patzer RE, McClellan WM, 2012. Influence of race, ethnicity and socioeconomic status on kidney disease. Nat. Rev. Nephrol 8 (9), 533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Rubio OMM, Amador JJ, Brooks DR, 2013. An epidemic of chronic kidney disease in Central America: an overview. J. Epidemiol. Community Health 67, 1–3. [DOI] [PubMed] [Google Scholar]
- Roncal-Jimenez CA, García-Trabanino R, Wesseling C, Johnson RJ, 2016. Mesoamerican nephropathy or global warming nephropathy? Blood Purif. 41 (1–3), 135–138. [DOI] [PubMed] [Google Scholar]
- Santos Ubiratan P, Zanetta DMT, Terra-Filho M, Burdmann EA, 2015. Burnt sugarcane harvesting is associated with acute renal dysfunction. Kidney Int. 87 (4), 792–799. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott KMMK, Kalandadze A, Li JY, Paragas N, Nicholas T, et al. , 2006. Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curricular Opin. Nephrol. Hypertens (15), 442–449. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott KMMK, Li JY, Kalandadze A, Cohen DJ, Devarajan P, et al. , 2007. Dual action of neutrophil gelatinase-associated lipocalin. J. Am. Soc. Nephrol 18, 407–413. [DOI] [PubMed] [Google Scholar]
- Schwartz JSJ, Patz JA, 2004. Hospital admissions for heart disease: the effects of temperature and humidity. Epidemiology 15, 755–761. [DOI] [PubMed] [Google Scholar]
- Semenza JC, McCullough JE, Flanders WD, McGeehin MA, Lumpkin JR, 1999. Excess hospital admissions during the July 1995 heat wave in Chicago. Am. J. Prev. Med 16 (4), 269–277. [DOI] [PubMed] [Google Scholar]
- Shankar A, Klein R, Klein BEK, 2006. The association among smoking, heavy drinking, and chronic kidney disease. Am. J. Epidemiol 164 (3), 263–271. [DOI] [PubMed] [Google Scholar]
- Sinharay S, Stern HS, Russell D, 2001. The use of multiple imputation for the analysis of missing data. Psychol. Methods 6 (4), 317–329. [PubMed] [Google Scholar]
- Smolander J, 2002. Effect of cold exposure on older humans. Int. J. Sports Med 23 (2), 86–92. [DOI] [PubMed] [Google Scholar]
- Song X, Wang S, Hu Y, et al. , 2017. Impact of ambient temperature on morbidity and mortality: an overview of reviews. Sci. Total Environ 586, 241–254. [DOI] [PubMed] [Google Scholar]
- Supavekin SZW, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P, 2003. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 63, 1714–1724. [DOI] [PubMed] [Google Scholar]
- Trabanino RGAR, Silva CR, Mercado MO, Merino RL, 2002. Nefropatía terminal en pacientes de un hospital de referencia en El Salvador. Rev. Panama de Salud Publica 12, 202–206. [DOI] [PubMed] [Google Scholar]
- Voss JD, Masuoka P, Webber BJ, Scher AI, Atkinson RL, 2013. Association of elevation, urbanization and ambient temperature with obesity prevalence in the United States. Int. J. Obes 37 (10), 1407–1412. [DOI] [PubMed] [Google Scholar]
- vWdJB Wesseling C., Crowe J, Rittner R, Sanati NA, Hogstedt C, Jakobsson K, 2015. Mesoamerican nephropathy: geographical distribution and time trends of chronic kidney disease mortality between 1970 and 2012 in Costa Rica. Occup. Environ. Med 72, 714–721. [DOI] [PubMed] [Google Scholar]
- Waite LJ, Laumann EO, Levinson W, Lindau ST, O’Muircheartaigh CA, 2014a. National Social Life, Health, and Aging Project (NSHAP): Wave 1 ICPSR20541-v6. Inter-university Consortium for Political and Social Research, Ann Arbor, MI, pp. 04–30 [distributor]. [Google Scholar]
- Waite LJCK, Dale W, Huang E, Laumann EO, McClintock M, et al. , 2014b. National Social Life, Health, and Aging Project (NSHAP): Wave 2 and Partner Data Collection ICPSR34921-v1. Inter-university Consortium for Political and Social Research, Ann Arbor, MI. [Google Scholar]
- Wang XYBA, Hu W, Tong S, 2009. Temperature variation and emergency hospital admissions for stroke in Brisbane, Australia, 1996–2005. Int. J. Biometeorol 53, 535–541. [DOI] [PubMed] [Google Scholar]
- Wesseling C, Aragón A, González M, et al. , 2016a. Kidney function in sugarcane cutters in Nicaragua – a longitudinal study of workers at risk of Mesoamerican nephropathy. Environ. Res 147, 125–132. [DOI] [PubMed] [Google Scholar]
- Wesseling C, Aragón A, González M, et al. , 2016b. Heat stress, hydration and uric acid: a cross-sectional study in workers of three occupations in a hotspot of Mesoamerican nephropathy in Nicaragua. BMJ Open 6 (12), e011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DD, Wong H, 2008. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit. Care Med 36 (4), 1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DS, Devarajan P, Ma Q, et al. , 2008. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit. Care Med 36 (4), 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IR, Royston P, Wood AM, 2011. Multiple imputation using chained equations: issues and guidance for practice. Stat. Med 30 (4), 377–399. [DOI] [PubMed] [Google Scholar]
- Woodhouse PR, Khaw KT, Plummer M, 1993. Seasonal variation of blood pressure and its relationship to ambient temperature in an elderly population. J. Hypertens 11 (11), 1267–1274. [PubMed] [Google Scholar]
- Yang J, Yin P, Zhou M, et al. , 2016. The effect of ambient temperature on diabetes mortality in China: a multi-city time series study. Sci. Total Environ 543, 75–82. [DOI] [PubMed] [Google Scholar]
- Yanosky JD, Paciorek CJ, Laden F, et al. , 2014. Spatio-temporal modeling of particulate air pollution in the conterminous United States using geographic and meteorological predictors. Environ. Health: A Glob. Access Sci. Source 13, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccali CM F, 2007. Adiponectin and renal disease progression: another epidemiologic conundrum? Kidney Int. 71 (12), 1195–1197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.