Figure 1.
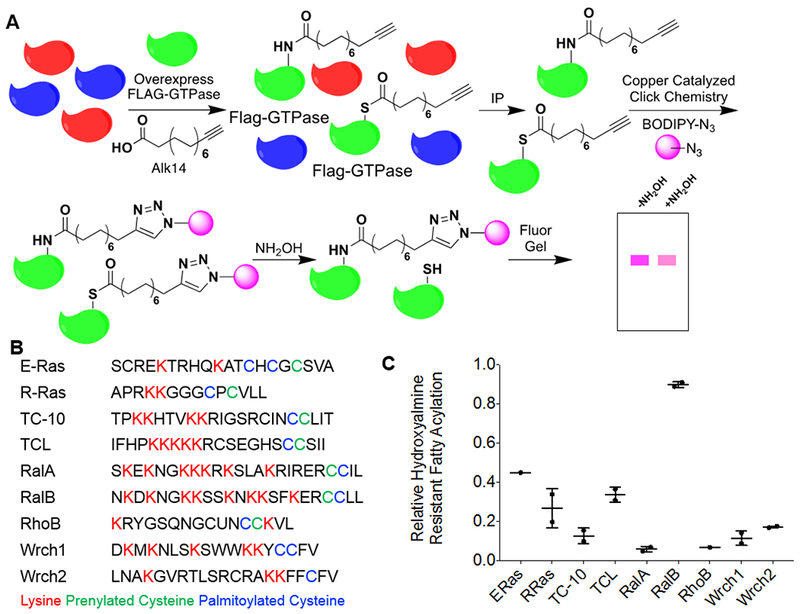
Several members of the Ras family small GTPases contain hydroxylamine-resistant fatty acylation. (A) The method used to detect lysine fatty acylation on proteins using the palmitic/myristic acid analogue, Alk14. N-terminal Flag-tagged small GTPases were overexpressed in HEK-293T cells. The cells were treated with 50 μM Alk14 (or the vehicle control DMSO) for 6 hours. After FLAG immunoprecipitation and on-bead click chemistry, fatty acylation levels were visualized using in-gel fluorescence with and without hydroxylamine treatment. (B) A list of related Ras family small GTPases and their respective c-terminal hypervariable region. Palmitoylated cysteine residues are highlighted in blue, prenylated cysteine residues are highlighted in green, and lysine residues that are potential sites for lysine fatty acylation are highlighted in red. (C) Quantification of the relative amount of hydroxylamine-resistant fatty acylation for each small GTPase. The fatty acylation level of each of the proteins listed was determined from two independent experiments (n=2). To quantify the relative hydroxylamine resistant fatty acylation, the fluorescence signal after hydroxylamine treatment, normalized to protein loading as determined by blue staining or a flag western blot, was divided by the fluorescence signal prior to hydroxylamine treatment, normalized to protein loading as determined by blue staining or a flag western blot.
