Abstract
Cryopreservation of ovarian tissue has been considered experimental for many years, but very recently the American Society of Reproductive Medicine is reviewing the process and perhaps soon will remove the label of “experimental” and recognize it as an established method for preserving female fertility when gonadotoxic treatments cannot be delayed or in patients before puberty or when there is desire to cryopreserve more than just few oocytes. This article discusses in detail the 3 methodologies used for cryopreservation: (a) slow freezing, (b) directional freezing, and (c) vitrification.
Keywords: Ovarian tissue, cryopreservation, whole ovary freezing
Introduction
Historically, the first successful freezing and re-transplant of ovarian tissue was performed and reported by Parkes and Smith.1 However, despite thousands of papers published on freezing of ovarian slices (Google Scholar—accessed February 2019), this landmark paper was cited only 37 times. Excellent reviews on the topic can be found in Donnez and many others.2-6
Fertility preservation is practiced today for women in their fertile years who for medical reasons (eg, undergoing chemotherapy or radiation) or life-style reasons (career choices, difficulty finding a partner) must postpone their time for pregnancy.7-12 Cryopreservation for cancer or medical reasons can rely on 3 main strategies used to safeguard women’s future fertility, consisting of (a) temporary pituitary ovarian downregulation by GnRHa to minimize chemotherapy-associated gonadotoxicity, (b) cryopreservation of ovarian cortical slices,13,14 or (c) vitrification of oocytes.15-19 Oocyte freezing is the main option chosen to electively postpone future fertility. Cryopreservation of ovarian tissue has been considered experimental for many years, but very recently the American Society of Reproductive Medicine (ASRM communication) is in the process of removing the label of “experimental” and recognize it as a method for preserving female fertility when gonadotoxic treatments cannot be delayed or in patients before puberty or when there is desire to cryopreserve more than just few oocytes.20,21 A very interesting recent paper compared oocytes versus ovarian tissue cryopreservation for women facing cancer.22
In this article, the 3 methodologies used for cryopreservation—slow freezing, directional freezing, and vitrification—will be discussed in detail.
Conventional Slow Freezing
In conventional slow freezing, the tissue is cooled to temperatures below the freezing point with or without seeding and then, at a controlled cooling rate, slowly frozen to a temperature between –35°C and –130°C before plunging into liquid nitrogen (LN).23 However, for many years, attempts to cryopreserve large tissue sections and whole organs have been ineffective because of problems associated with heat and mass transfer.24 The heat transfer between the outer area and the center of large tissue sections creates nonhomogeneous rates of cooling between the core and the periphery.25 In addition, large biological samples suffer from the long isothermal period caused by the massive release of latent heat occurring during the process of ice formation.24,26 The phenomenon of latent heat is caused by the energy generated by water molecules when they rejoin to form ice crystals. Such energy is released in the form of heat, which, in turn, causes a temperature rise of the surrounding structures.27 The heat is normally transferred to the ice crystals just formed because they are made of conductive material. The consequence of this physical process is a transient thawing followed by refreezing, in a sequence that is repeated several times through the thickness of the tissue sample, causing severe cell damage.28 Damages are usually reduced by keeping the ratio of surface to volume as high as possible, so that the excessive latent heat is removed by adjusting the cooling rate with the intent to achieve a uniform freezing. The smaller the sample, the faster the heat released from its inner part is removed, thus minimizing damages. However, the need of samples as small as possible is in contrast with the requirement of having a well-preserved large follicle population on thawing. It is also impossible to reduce the surface-to-volume ratio in samples like entire organs such as whole ovaries. Therefore, new strategies had to be implemented. The problem with mass transfer must be dealt both before freezing and, later, after thawing. Before freezing, the use of cryoprotectant (CP) solutions capable of penetrating in the tissue to a sufficient depth to reach every single cell in the tissue creates protection from ice damages. After thawing, the CP solutions must then be removed prior to transplantation because they can be toxic once the tissue or organ is at physiological temperature.
Vitrification
Vitrification is a process in which ice crystal formation is avoided by exposing tissue or cells to high concentrations of CPs followed by rapid cooling by plunging into LN or in LN slush at temperatures of –210°C.29 For many years, slow freezing, and not vitrification, was the method of choice for embryo cryopreservation. In 1985, the first successful vitrification of mouse embryos using a relatively large volume sample was reported.30 As stated above, vitrification is the process in which a sample solidifies without the formation of ice crystals, thus resulting in a glassy amorphous state. The main factors that influence the probability for vitrification to occur are as follows:
Sample’s volume—the lower the volume, the greater the chances for vitrification to occur.
Cooling rate—as the cooling rate increases, the chances for ice to grow into large crystals decreases.
Sample’s viscosity—the higher the viscosity of the sample, the higher are the chances to avoid ice crystallization (see Arav equation)
In 1989, the “minimum drop size” (MDS) method was developed by Arav.19,31 However, vitrification as a methodology for preserving large biological samples such as vascularized organs has many drawbacks: chemical toxicity and osmotic shock following exposure to very high (>50%) concentrations of CP solutions, fractures caused to the solidified organ by the vitrification procedure,32 and devitrification if the storage temperature is above the glass transition temperature.33 Vitrification is now the method of choice for preserving oocytes and embryos, but it is slowly gaining acceptance also for the gonadal tissue. However, the procedure is cumbersome, requires highly skilled personal, and is not standardized, thus producing variable results. We have recently developed a device for automatic vitrification of oocytes, embryos, and ovarian and testicular tissue34 (see Figure 1). Having an automated device allowing the precise exposure of the tissue to cooling and warming solutions is desirable, particularly for preserving fertility for cancer patients. The device is fully automated (Sarah; FertileSafe Ltd, Israel) for both vitrification and warming of gonadal tissue slices in addition to oocytes and embryos.34 A simplified, semi-automatic method has been recently introduced in the veterinary field (Ledda et al in press JASB 2019) using a special straw (called E.Vit) which stands for easy vitrification and is under the regulation review process for the human field.
Figure 1.

Schematic representation of the “Sarah” (FertileSafe Ltd, Israel) device for automation of the freezing process.
Warming of Vitrified Tissue
To complete a successful cycle of vitrification/rewarming, it is extremely important to reach high warming rates. However, this is difficult to achieve in large tissue and organs.33 The physical phenomenon of “heat transfer” is the limiting key factor that occurs during conventional warming (eg, when exposing vitrified tissue/cells to water bath). The slow warming rate is deleterious causing the formation of ice crystal growth during the warming stage (devitrification). Etheridge and colleagues35 showed recently a new approach for rapidly and uniformly producing heating using radiofrequency (RF) excited by magnetic nanoparticles. We also showed a benefit of the RF system by applying the new concept of RF feedback technology.36 In a simulation experiment, the new RF heating technology has been shown to be able to uniformly increase the warming rate of 150 mL of the solution mimicking a large size of tissue (Figure 2).
Figure 2.
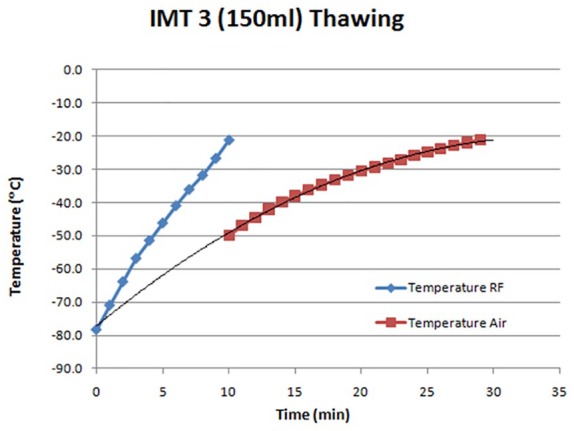
Warming 150 mL of IMT (IMT Ltd Ness ziona, Israel) solution by RF heater. RF indicates radiofrequency.
Directional Freezing
An alternative technique to slow cooling is known as directional solidification or directional freezing. After the initial seeding stage (which is done automatically in the same device), the tissue sample (see Figure 3) is advanced at a constant velocity through a linear temperature gradient. This allows precise control over the freezing process, facilitating very accurate and homogeneous cooling of the tissue, with a controlled ice crystal growth, while the tissue sample is moved down the temperature gradient. Dissipation of heat from the sample is achieved primarily in 2 ways. The highly heat-conductive metal block surrounding the sample removes efficiently the heat away from the biological sample as it freezes. Because of the temperature gradient and the directional forward movement of the sample, the heat is continuously removed from the ice front into the unfrozen fraction of the sample that is still located at the higher temperature end of the thermal gradient. This controlled and directional heat dissipation protects the ice front from melting and refreezing, thus avoiding damages to the cells. All these processes result in highly controlled and “cell-friendly” ice crystal morphology that contributes significantly to reduce mechanical damages.38-40
Figure 3.
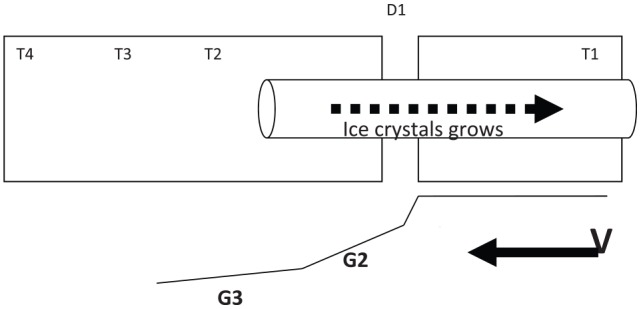
Schematic principle of the “multi-thermal gradient” (MTG) freezing technology. After the initial seeding stage, the tissue sample is loaded and placed into the device (T1) and advanced at a constant velocity (V) through a linear temperature gradient (G1-G3).37
The efficient heat removal and the precisely controlled ice crystal propagation make it possible to use this technology to freeze small and large volumes alike.41
As discussed, the cryopreservation of ovarian tissue is now a well-established method for preserving female fertility.20,21 This can be achieved through the excision and banking of the entire organ or of fragments of the ovarian cortex. However, whereas no live births or pregnancies have been achieved following the cryopreservation of whole human ovaries, more than 130 births have been obtained after re-transplantation of frozen-thawed ovarian cortical fragments.6 This clearly suggests that the use of cortical fragments is the method of choice over whole ovary preservation.3,41 We have recently performed a direct comparison of sheep ovarian tissue viability between organs cryopreserved with a conventional programmable slow freezer or a directional freezing apparatus.42,43 Our results indicate that ovarian structures and functions were significantly better preserved by directional freezing.
The morphological analysis of ovarian tissue immediately after thawing showed that the use of directional freezing significantly improved the integrity of all follicular structures from primordial to secondary stages. In addition, consistent with the favorable effect of directional freezing on primordial follicle viability in vitro, we observed a higher cell proliferation rate compared with samples that underwent conventional freezing. However, so far, no clinical studies have been conducted with directional freezing of ovarian slices.
Freezing Large Organs: The Whole Ovary
One of the main drawbacks of ovarian tissue freezing and transplantation is the warm ischemia of the graft following transplantation.
In theory, the cryopreservation of whole ovaries followed by vascular anastomosis of the ovarian pedicle of the thawed organ should provide a larger follicular reserve and a longer life span of the transplant. However, currently available data do not support this hypothesis. This is largely due to the damages caused by the freezing procedures applied to a sample as large as a human ovary. We cryopreserved the whole ovary of sheep44,45 and human46,47 using directional freezing. In a comparative study on sheep, we found that directional freezing improves the viability of cryopreserved ovarian tissue when used not only with whole organs but also with ovarian fragments.42,43 The direct comparison of cortical fragments and whole organs unexpectedly revealed that the latter show a better preservation of early follicles in many aspects almost identical to those recorded in fresh control samples. However, the persistent technical difficulties linked to the surgical procedures required for re-transplantation are likely to still limit the use of whole ovaries in clinical settings. One more possibility is to freeze the whole ovary and to transfer cortical slices following the thawing.
Transplantation of the fresh whole ovary has been attempted with some success both in several animal species48-51 and in human,9,41 demonstrating that anastomosis of the ovarian pedicle is difficult but feasible. However, cryopreservation of the entire organ has proved difficult, largely due to the physical constraints that limit an appropriate heat transfer between the core and the periphery of the organ.38 In addition, the large volume of the whole organ poses some limitations to the perfusion and diffusion of CPs.52 Both are essential for preventing intravascular ice formation which would irreversibly compromise a rapid and efficient resumption of the blood supply.26 An efficient protocol for the cryopreservation of the entire ovary would provide a possible way to improve the ischemic damages observed when avascular fragments are transplanted. However, current data obtained from experiments mostly performed in sheep do not support this hypothesis. Despite some positive results53,54 and pregnancies,55 stromal and vascular damages of variable extent were observed both following slow freezing56 as well as after vitrification.31
To move forward ovarian cryopreservation, in the case of both avascular ovarian fragments and whole organs, requires an improvement of the freezing techniques.20 A direct comparison between a conventional programmable slow freezer and the multi-thermal gradient (MTG) apparatus for the cryopreservation of sheep ovarian cortical strips and of whole ovaries showed, in all experiments, that the MTG method was superior for large organ freezing.38,42,43
Footnotes
Declaration of conflicting interests:A.A. and P.P. are co-founders of FertileSafe Ltd, Israel.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: AA and PP contributed equally to this article.
ORCID iD: Pasquale Patrizio  https://orcid.org/0000-0003-4796-7078
https://orcid.org/0000-0003-4796-7078
References
- 1. Parkes AS, Smith AU. Regeneration of rat ovarian tissue grafted after exposure to low temperatures. Proc R Soc Lond B Biol Sci. 1953;140:455-470. [DOI] [PubMed] [Google Scholar]
- 2. Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12:519-535. [DOI] [PubMed] [Google Scholar]
- 3. Donnez J, Dolmans MM. Fertility preservation in women. Nat Rev Endocrinol. 2013;9:735-749. [DOI] [PubMed] [Google Scholar]
- 4. Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30:11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dolmans MM, Jadoul J, Gilliaux G, et al. A review of 15 years of ovarian tissue bank activities. J Assist Reprod Genet. 2013;30:305-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Donnez J, Dolmans M-M. Fertility preservation in women. N Engl J Med. 2017;377:1657-1665. [DOI] [PubMed] [Google Scholar]
- 7. Cobo A, Domingo J, Perez S, Crespo J, Remohi J, Pellicer A. Vitrification: an effective new approach to oocyte banking and preserving fertility in cancer patients. Clin Transl Oncol. 2008;10:268-273. [DOI] [PubMed] [Google Scholar]
- 8. Gosden R. Cryopreservation: a cold look at technology for fertility preservation. Fertil Steril. 2011;96:264-268. [DOI] [PubMed] [Google Scholar]
- 9. Silber SJ. Fresh ovarian tissue and whole ovary transplantation. Semin Reprod Med. 2009;27:479-485. [DOI] [PubMed] [Google Scholar]
- 10. Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2011;18:59-67. [DOI] [PubMed] [Google Scholar]
- 11. Stoop D, Cobo A, Silber S. Fertility preservation for age-related fertility decline. Lancet. 2014;384:1311-1319. [DOI] [PubMed] [Google Scholar]
- 12. Inhorn MC, Birenbaum-Carmeli D, Birger J, et al. The socio-demographic of elective egg freezing: a binational analysis of fertility preservation among unpartnered healthy women. RBE. 2018;16:70-81. [Google Scholar]
- 13. Gosden RG, Baird DT, Wade JC. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at -196 degrees C. Hum Reprod. 1994;9:597-603. [DOI] [PubMed] [Google Scholar]
- 14. Andersen CY, Silber SJ, Bergholdt SH, Jorgensen JS, Ernst E. Long-term duration of function of ovarian tissue transplants: case reports. Reprod Biomed Online. 2012;25:128-132. [DOI] [PubMed] [Google Scholar]
- 15. Cobo A, Garcia-Velasco JA, Domingo J, Remohi J, Pellicer A. Is vitrification of oocytes useful for fertility preservation for age-related fertility decline and in cancer patients. Fertil Steril. 2013;99:1485-1495. [DOI] [PubMed] [Google Scholar]
- 16. Nagy ZP, Chang CC, Shapiro DB, et al. Clinical evaluation of the efficiency of an oocyte donation program using egg cryo-banking. Fertil Steril. 2009;92:520-526. [DOI] [PubMed] [Google Scholar]
- 17. Noyes N, Labella PA, Grifo J, Knopman JM. Oocyte cryopreservation: a feasible fertility preservation option for reproductive age cancer survivors. J Assist Reprod Genet. 2010;27:495-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rienzi L, Cobo A, Paffoni A, et al. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Hum Reprod. 2012;27:1606-1612. [DOI] [PubMed] [Google Scholar]
- 19. Arav A. Vitrification of oocyte and embryos. In: Lauria A, Gandolfi F, eds. New Trends in Embryo Transfer. Cambridge, UK: Portland Press; 1992:255-264. [Google Scholar]
- 20. Donnez J, Dolmans MM, Pellicer A, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99:1503-1513. [DOI] [PubMed] [Google Scholar]
- 21. Grynberg M, Poulain M, Sebag-Peyrelevade S, le Parco S, Fanchin R, Frydman N. Ovarian tissue and follicle transplantation as an option for fertility preservation. Fertil Steril. 2012;97:1260-1268. [DOI] [PubMed] [Google Scholar]
- 22. Diaz-Garcia C, Domingo J, Garcia-Velasco JA, et al. Oocyte vitrification versus ovarian cortex transplantation in fertility preservation for adult women undergoing gonadotoxic treatments: a prospective cohort study. Fertil Steril. 2018;109:478.e2-485.e2. [DOI] [PubMed] [Google Scholar]
- 23. Morris GJ, Acton E, Faszer K, et al. Cryopreservation of murine embryos, human spermatozoa and embryonic stem cells using a liquid nitrogen-free, controlled rate freezer. Reprod Biomed Online. 2006;13:421-426. [DOI] [PubMed] [Google Scholar]
- 24. Balasubramanian SK, Coger RN. Heat and mass transfer during the cryopreservation of a bioartificial liver device: a computational model. ASAIO J. 2005;51:184-193. [DOI] [PubMed] [Google Scholar]
- 25. Koebe HG, Werner A, Lange V, Scildberg FW. Temperature gradients in freezing chambers of rate-controlled cooling machines. Cryobiology. 1993;30:349-352. [Google Scholar]
- 26. Pegg D. The relevance of ice crystal formation for the cryopreservation of tissues and organs. Cryobiology. 2010;60:S36-S44. [DOI] [PubMed] [Google Scholar]
- 27. Gavish Z, Ben-Haim M, Arav A. Cryopreservation of whole murine and porcine livers. Rejuvenation Res. 2008;11:765-772. [DOI] [PubMed] [Google Scholar]
- 28. Koshimoto C, Mazur P. Effects of warming rate, temperature, and antifreeze proteins on the survival of mouse spermatozoa frozen at an optimal rate. Cryobiology. 2002;45:49-59. [DOI] [PubMed] [Google Scholar]
- 29. Arav A, Yavin S, Zeron Y, Natan Y, Dekel I, Gacitua H. New trend in gamete’s cryopreservation. Mol Cell Endocrinology. 2002;187:77-81. [DOI] [PubMed] [Google Scholar]
- 30. Fahy GM, Rall WF. Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature. 1985;313:573-575. [DOI] [PubMed] [Google Scholar]
- 31. Arav A. Vitrification of Oocytes and Embryos [DVM thesis]. Bologna, Italy: Bologna University; 1989. [Google Scholar]
- 32. Courbiere A, Massardier J, Salle B, Mazoyer C, Guerin JF, Lornage J. Follicular viability and histological assessment after cryopreservation of whole sheep ovaries with vascular pedicle by vitrification. Fertil Steril. 2005;84:1065-1071. [DOI] [PubMed] [Google Scholar]
- 33. Fahy G, MacFarlane D, Angell C. Vitrification as an approach to cryopreservation. Cryobiology. 1984;21:407-426. [DOI] [PubMed] [Google Scholar]
- 34. Arav A, Natan Y, Kalo D, et al. A new, simple, automatic vitrification device: preliminary results with murine and bovine oocytes and embryos. J Assist Reprod Genet. 2018;35:1161-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Etheridge ML, Xu Y, Rott L, Choi J, Glasmacher B, Bischof JC. RF heating of magnetic nanoparticles improves the thawing of cryopreserved biomaterials. Technology. 2014;2:229-242. [Google Scholar]
- 36. Arav A, Natan Y. Freeze drying of red blood cells: the use of directional freezing and a new radio frequency lyophilization device. Biopreservat Biobank. 2011;10:386-394. [DOI] [PubMed] [Google Scholar]
- 37. Arav A. Device and methods for multigradient directional cooling and warming of biological samples. US Patent 5873254A. February 23, 1999. [Google Scholar]
- 38. Arav A, Natan Y. Directional freezing: a solution to the methodological challenges to preserve large organs. Semin Reprod Med. 2009;27:438-442. [DOI] [PubMed] [Google Scholar]
- 39. Arav A, Gavish Z, Elami A, et al. Ovarian function 6 years after cryopreservation and transplantation of whole sheep ovaries. Reprod Biomed Online. 2010;20:48-52. [DOI] [PubMed] [Google Scholar]
- 40. Arav A, Natan D. Directional freezing of reproductive cells and organs. Reprod Domest Anim. 2012;47:193-196. [DOI] [PubMed] [Google Scholar]
- 41. Bromer JG, Patrizio P. Fertility preservation: the rationale for cryopreservation of the whole ovary. Semin Reprod Med. 2009;27:465-471. [DOI] [PubMed] [Google Scholar]
- 42. Maffei S, Hanenberg M, Pennarossa G, et al. Direct comparative analysis of conventional and directional freezing for the cryopreservation of whole ovaries. Fertil Steril. 2013;100:1122-1131. [DOI] [PubMed] [Google Scholar]
- 43. Maffei S, Pennarossa G, Brevini TA, Arav A, Gandolfi F. Beneficial effect of directional freezing on in vitro viability of cryopreserved sheep whole ovaries and ovarian cortical slices. Hum Reprod. 2013;29:114-124. [DOI] [PubMed] [Google Scholar]
- 44. Revel A, Elami A, Bor A, et al. Whole organ cryopreservation, successful transplantation of sheep ovary frozen at -196°C. Fertil Steril. 2004;82:1714-1715. [DOI] [PubMed] [Google Scholar]
- 45. Arav A, Revel A, Nathan Y, et al. Oocyte recovery, embryo development and ovarian function after cryopreservation and transplantation of whole sheep ovary. Hum Reprod. 2005;20:3554-3559. [DOI] [PubMed] [Google Scholar]
- 46. Patrizio P, Gavish Z, Martel M, Azodi M, Silber S, Arav A. Whole human ovaries cryopreservation using a novel multi-gradient freezing device. Fertil Steril. 2007;88:S355. [Google Scholar]
- 47. Kallen A, Patrizio P. The challenges and promises of whole human ovary cryopreservation. In: Bedawy M, Rizk B, eds. Fertility Preservation: Advances and Controversies. New Delhi, India: Jaypee Brothers Medical Publishers; 2014:96-101. [Google Scholar]
- 48. Hilders CG, Baranski AJ, Peters L, Ramkhelawan A, Trimbos JB. Successful human ovarian autotransplantation to the upper arm. Cancer. 2004;101:2771-2778. [DOI] [PubMed] [Google Scholar]
- 49. Leporrier M, Von Theoblad P, Roffe JL, Muller G. A new technique to protect ovarian function before pelvic irradiation: heterotopic ovarian autotransplantation. Cancer. 1987;60:2201-2204. [DOI] [PubMed] [Google Scholar]
- 50. Bedaiwy MA, Jeremias E, Gurunluoglu R, et al. Restoration of ovarian function after autotransplantation of intact frozen-thawed sheep ovaries with microvascular anastomosis. Fertil Steril. 2003;79:594-602. [DOI] [PubMed] [Google Scholar]
- 51. Arav A, Gavish Z, Elami A, Revel A, Gosden RG, Patrizio P. A six-year record of ovarian function after orthotopic vascular transplantation of whole cryopreserved sheep ovaries. Reprod Biomed Online. 2010;20:48-52. [DOI] [PubMed] [Google Scholar]
- 52. Grazul-Bilska AT, Banerjee B, Yazici I, et al. Morphology and function of cryopreserved whole ovine ovaries after heterotopic autotransplantation. Reprod Biol Endocrinol. 2008;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Milenkovic M, Diaz-Garcia C, Wallin A, Brannstrom M. Viability and function of the cryopreserved whole rat ovary: comparison between slow-freezing and vitrification. Fertil Steril. 2012;97:1176-1182. [DOI] [PubMed] [Google Scholar]
- 54. Onions VJ, Webb R, McNeilly AS, Campbell BK. Ovarian endocrine profile and long-term vascular patency following heterotopic autotransplantation of cryopreserved whole ovine ovaries. Hum Reprod. 2009;24:2845-2855. [DOI] [PubMed] [Google Scholar]
- 55. Salle B, Demirci B, Franck M, Rudigoz RF, Guerin JF, Lornage J. Normal pregnancies and live births after autograft of frozen-thawed hemiovaries into ewes. Fertil Steril. 2002;77:403-408. [DOI] [PubMed] [Google Scholar]
- 56. Wallin A, Ghahremani M, Dahm-Kahler P, Brannstrom M. Viability and function of the cryopreserved whole ovary: in vitro studies in the sheep. Hum Reprod. 2009;24:1684-1694. [DOI] [PubMed] [Google Scholar]


