Abstract
Background:
The primary objective of this study was to analyze the cross-reactivity of antidrug antibodies to reference adalimumab (ADL) and SB5 (adalimumab biosimilar) in patients with inflammatory bowel disease (IBD) or rheumatoid arthritis (RA).
Methods:
Sera from patients with IBD and RA with or without antibodies to adalimumab (ATA+ or ATA–, respectively) were tested for cross-reactivity with SB5 and ADL. Functional inhibition of tumor necrosis factor-α binding was measured. Sera from patients with antibodies to reference infliximab (ATI+) were examined for cross-reactivity to SB5. Sera were tested by enzyme-linked immunosorbent assay.
Results:
All 30 anti-ADL ATA+ sera from patients with IBD and all 4 anti-SB5 ATA+ sera from patients with RA were cross-reactive with ADL and SB5 (range of mean concentrations: IBD, 20.99–21.31 μg/ml; RA, 16.46–17.48 μg/ml). In general, there was no significant difference between mean ATA titers. A strong correlation was detected in all ATA+ samples (rho = 0.997 to >0.999; p < 0.001 each). However, ATA– sera were not reactive to either ADL or SB5. anti-ADL ATA+ sera similarly neutralized functional activity of ADL and SB5; no functional inhibition was observed with ATA– sera. ATI+ sera did not cross-react with SB5.
Conclusions:
ADL and SB5 show cross-immunogenicity in sera from patients with IBD or RA, supporting shared immune-dominant epitopes. ATI+ sera did not cross-react with SB5, suggesting different immunogenic epitopes between infliximab and SB5.
Keywords: anti-TNF, biologic therapies, biosimilar, adalimumab, inflammatory bowel disease
Introduction
Biosimilars are therapeutic agents that are similar to, and demonstrate no clinically meaningful differences from, approved biologic agents.1 To be approved by regulatory agencies, biosimilars must demonstrate similarity in terms of quality, critical physicochemistry, and biological attributes.2,3 Biosimilars must also show equivalent efficacy and safety to the reference product in clinical trials, which includes pharmacokinetic (PK) and pharmacodynamic properties,4,5 and/or an immunogenicity profile similar to the reference product.2,3 Clinical equivalence studies are generally performed in the most sensitive indications, such as rheumatoid arthritis (RA) and psoriasis, to detect even minor differences between the reference biologic and the biosimilar, and to support extrapolation to other indications.6 Although clinical trials are required for demonstrating efficacy, in vitro assays have higher sensitivity for identifying differences in biological activity.6 These in vitro assays include demonstration of characteristics relevant to the mechanism of action of the reference product, such as tumor necrosis factor-alpha (TNF-α) neutralization and binding affinity, among others.6
The biosimilar SB5 (Imraldi®, Samsung Bioepis Co., Ltd., Incheon, Republic of Korea) is approved by the European Commission for the same indications as reference adalimumab (ADL), including RA, juvenile idiopathic arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis, pediatric plaque psoriasis, hidradenitis suppurativa, inflammatory bowel disease (IBD; i.e. Crohn disease, pediatric Crohn disease, ulcerative colitis), and uveitis.7 The approval of SB5 was based on the results of physicochemical,8 biological,8,9 and clinical evaluation.10 In a phase III, randomized, double-blind, parallel-group study, SB5 and ADL demonstrated comparable efficacy, safety, PK, and immunogenicity in patients with moderate-to-severe RA.10 Physicochemical characterization showed that SB5 and ADL had identical primary sequences and were highly similar in terms of secondary and tertiary structure, post-translational modifications, and purity/impurity profile.8 Biological characterization further showed that SB5 and ADL exhibited similar binding of TNF-α and neutralization of cytokine effects, as well as similar binding of various Fcγ receptors and Fc-related effector functions.8,9
Although SB5 and ADL have extensive comparative data, including comparable immunogenicity in the phase III RA study,10 data on immunogenicity and cross-switching in IBD are lacking, and antidrug antibody development can potentially lead to higher drug clearance, and, therefore, reduced efficacy in this population.6 As a result, more information is needed in terms of cross-immunogenicity. Cross-immunogenicity testing assay is an in vitro assay to evaluate whether antibodies against anti-TNF produced by patients can bind and functionally inhibit anti-TNF biosimilar or vice versa.11–13 The objectives of this study were to analyze cross-reactivity of antidrug antibodies to SB5 in patients with IBD who had previously been treated with ADL and cross-reactivity of antidrug antibodies to ADL in patients with RA who had previously been treated with SB5. Supportive analyses examined functional inhibition of TNF-α binding of SB5 and ADL and cross-reactivity of antidrug antibodies to SB5 in patients with IBD who had previously been treated with reference infliximab (IFX).
Methods
Patient samples
Sera were collected from patients with IBD treated with ADL or INF at a single medical center in Israel. Sera from patients with RA who participated in phase III clinical trials of SB5 were also included, as were sera from healthy donors. One serum sample was analyzed per patient or donor in duplicate. This study was conducted in accordance with the Declaration of Helsinki, and was approved by the IRB committee of Tel-Hashomer Medical Center (approval number 5598-08). All patients and healthy donors provided written informed consent.
Assessment of antibody concentration
Sera were tested using a drug-tolerant, enzyme-linked immunosorbent assay (ELISA), with a cut-off level of 2.3 μg/ml for antibody-to-adalimumab (ATA) detection. Wells of the ELISA plate were coated with 500 ng/ml TNF-α in bicarbonate buffer (100 μl/well) and incubated overnight at 4°C. Plates were then washed two times with 250 μl of 0.05% Tween-20 in phosphate-buffered saline (PBS) before being blocked with 1% (v/v) bovine serum albumin (BSA) in PBS (150 μl/well) overnight at 4°C or for 1 h at room temperature. ADL or SB5 (50 mg/ml; two different lots for each drug) in 1% BSA in PBS (diluted in 1:1000 with final concentration 50 μg/ml) was added to each well (100 μl/well), and the plate was incubated for 1 h at room temperature with shaking at 200 rpm. Wells were then washed three times with 250 μl of 0.05% Tween-20 in PBS. Serum samples were diluted 1:50 and 1:100 in the wells, and incubated for 1 h at room temperature at 200 rpm. Wells were then washed four times with 250 μl of 0.05% Tween-20 in PBS. Anti-Fab2-HRP standards were prepared by starting with 600 ng/ml, then two-fold serial dilution to have eight points of concentrations. Appropriately diluted anti-Fab2-HRP standards were added to each well in triplicate. Anti-lambda-HRP antibody (100 μl/well) was added to wells containing sample, and the plate was incubated for 1 h at room temperature at 200 rpm. Wells were then washed four times with 250 μl of 0.05% Tween-20 in PBS. Then, 100 μl TMB ELISA substrate was added to each well, and the sample was incubated until it turned a blue-turquoise color (~6–8 min). To stop the reaction, 50 μl of 2 M H2SO4 was added to each well. The plate was read on an ELISA plate reader at 450/540 nm in 30 min. The assay was determined to be optimal when the R2 was between 0.98 and 0.99 and the anti-Fab2-HRP standard of 600 ng/ml was above an optical density of 0.8.
The same procedure was used for antibody-to-INF (ATI) detection except that infliximab concentration used for antibody detection was 100 μg/ml instead of 50 μg/ml (adalimumab concentration used for antibody detection). The cut-off value of ATI detection was 2.5 μg/ml.
ATA cross-reactivity with ADL and SB5 in IBD
Cross-reactivity was determined in 30 sera samples that were positive for anti-ADL ATA (ATA+) from 30 patients with IBD treated with ADL and in 23 sera samples that were negative for ATA (ATA–). The ATA– samples included 10 sera samples from 10 patients with IBD treated with ADL and 13 samples from 13 healthy donors (negative control). Testing was performed using a validated, sensitive anti-lambda chain semi-quantitative ELISA, as described above.
ATA cross-reactivity with ADL and SB5 in RA
Cross-reactivity was determined in 4 anti-SB5 ATA+ sera samples from 4 patients with RA treated with SB5 and in 28 ATA– samples, including 4 samples from four patients with RA treated with SB5 and 24 samples from 24 patients with IBD treated with ADL (negative control). ATA+ and ATA– sera were tested by ELISA, as described above.
Functional inhibition of SB5 and ADL in ADL-treated patients with IBD
Five anti-ADL ATA+ sera from five patients with IBD were tested for their ability to inhibit TNF binding using graded concentrations of exogenous SB5 compared with similar concentrations of ADL. Five ATA– sera from five healthy donors served as negative controls. Sera were pre-incubated with the designated concentrations of exogenous ADL or SB5 for 30 min at room temperature. Sera were then added to plates precoated with TNF-α. Following incubation and washing, the amount of bound ADL or SB5 was determined using the anti-lambda ELISA, as a measure of the degree of neutralization of ADL-TNF binding capacity by the respective sera.
Cross-reactivity of antibodies to infliximab with SB5 in patients with IBD
To determine if ATI cross-react with SB5, 11 ATI+ sera from 11 patients with IBD previously exposed to INF, 5 anti-ADL ATA+ sera from 5 patients with IBD previously exposed to ADL, and 4 negative control sera from four healthy controls were reacted with two lots of SB5. The amount of bound antidrug antibodies was detected by ELISA.
Statistical analyses
Cross-reactivity with ADL and SB5 was tested by comparison of mean concentrations and significance was determined using a paired t test (if normality was accepted by Shapiro-Wilk test) or Wilcoxon test (if normality was rejected). For a supportive analysis, a two-way analysis of variance (ANOVA) with repeated measures was performed. Correlation coefficients were determined using Spearman correlation tests. All calculations were performed using MedCalc statistical software version 16.8.4 (MedCalc Software bvba, Ostend, Belgium). Results were considered significant if p < 0.05.
Results
Cross-reactivity in patients with IBD
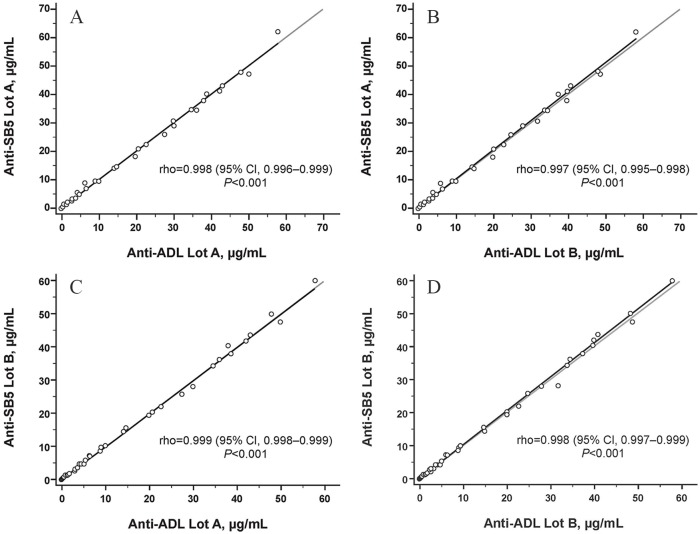
All 30 anti-ADL ATA+ sera from ADL-treated patients with IBD were cross-reactive with SB5. The mean (SD) concentration for anti-ADL ATA+ sera measured against SB5 lot A (original lot number 030G15), SB5 lot B (original lot number 029G15), ADL lot A (original lot number 1055417), and ADL lot B (original lot number 1042000) was 21.31 (16.96), 21.26 (17.05), 21.30 (17.07), and 20.99 (16.88) μg/ml, respectively (Table 1; Figure 1). When analyzed using a paired t test or Wilcoxon test, there was no significant difference between mean ATA titers measured against either SB5 or ADL lot, with the exception of SB5 lot B and ADL lot B (p = 0.047; Table 1). When analyzed using a two-way ANOVA, there was no significant difference between mean ATA titers measured against either SB5 or ADL lot. A strong correlation was detected between titers of ATA to both lots of SB5 and ADL (rho = 0.997–0.999; p < 0.001 each; Figure 2).
Table 1.
Comparative ATA titers toward the designated SB5 and ADL lots among anti-ADL ATA+ and ATA– sera from patients with inflammatory bowel disease.
| SB5 |
ADL |
|||
|---|---|---|---|---|
| Sera type | Lot A | Lot B | Lot A | Lot B |
| ATA+, mean (SD) | 21.31 (16.96) | 21.26 (17.05) | 21.30 (17.07) | 20.99 (16.88) |
| ADL lot A, p value* for difference between lots | NS | NS | – | – |
| ADL lot B, p value for difference between lots | NS | 0.0427 | – | – |
| ATA–, mean (SD) | 0.84 (0.75) | 0.82 (0.74) | 0.72 (0.70) | 0.71 (0.72) |
| ADL lot A, p value* for difference between lots | <0.05 | <0.05 | – | – |
| ADL lot B, p value for difference between lots | <0.05 | <0.05 | – | – |
If normality was accepted (Shapiro-Wilk test), parametric methods were used for analysis; mean of difference between two lots and p value by paired t test were calculated.
ADL, reference adalimumab; ATA, antidrug antibody to adalimumab; NS, not significant.
Figure 1.
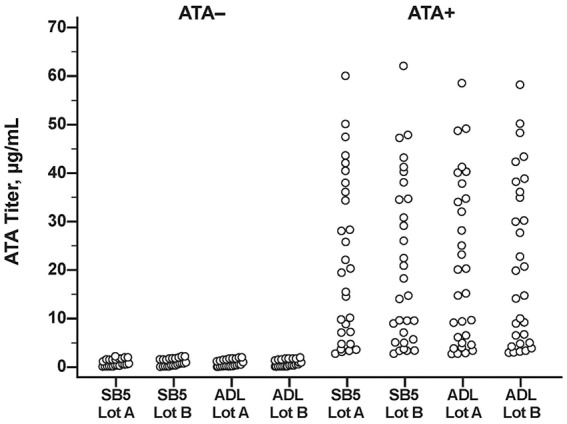
Comparative ATA titers toward the designated SB5 and ADL lots among anti-ADL ATA+ and ATA– sera from patients with inflammatory bowel disease.
ADL, reference adalimumab; ATA, antidrug antibody to adalimumab; ATA+, positive for ATA; ATA–, negative for ATA.
Figure 2.
Correlation between ATA concentrations from patients with inflammatory bowel disease treated with ADL or from negative controls, measured using the designated SB5 or ADL lots as the antigen for serum immune-reactivity. Correlation coefficient determined using Spearman correlation test.
ADL, reference adalimumab; ATA, antidrug antibody to adalimumab.
None of the 23 ATA– sera were reactive to either lot of SB5 or ADL. The mean (SD) concentration for ATA– sera measured against SB5 lot A, SB5 lot B, ADL lot A, and ADL lot B was 0.84 (0.75), 0.82 (0.74), 0.72 (0.70), and 0.71 (0.72) μg/ml, respectively (Table 1; Figure 1). When analyzed using the Wilcoxon test, there was a significant difference between mean ATA titers measured against each lot of SB5 and ADL (Table 1), but the absolute difference between the means was 0.10 μg/ml, which was within the negative background signal range of the assay. Similar results were seen when analyzed using a two-way ANOVA.
Cross-reactivity in patients with RA
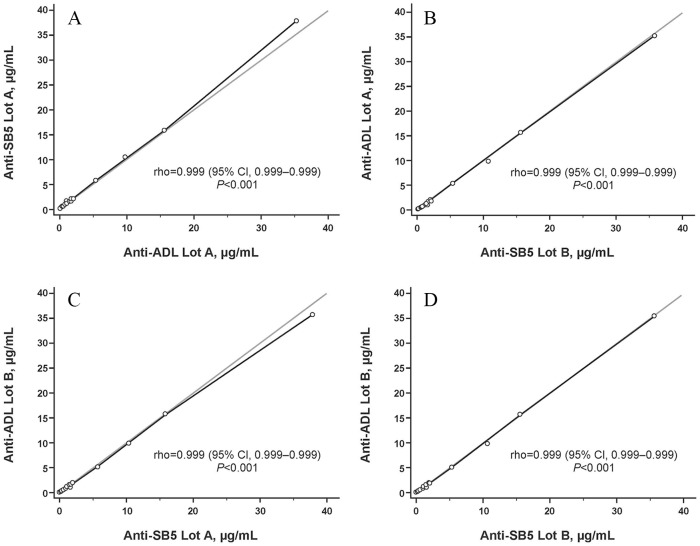
All four anti-SB5 ATA+ sera from SB5-treated patients with RA were cross-reactive with ADL. Mean (SD) concentrations for anti-SB5 ATA+ sera measured against SB5 lot A, SB5 lot B, ADL lot A, and ADL lot B were 17.48 (14.24), 16.88 (13.36), 16.46 (13.19), and 16.60 (13.45) μg/ml, respectively (Table 2; Figure 3). There was no significant difference in mean ATA titers measured against either lot of SB5 or ADL when analyzed using a paired t test, Wilcoxon test, or two-way ANOVA (Table 2). A strong correlation was detected between titers of ATA to both lots of SB5 and ADL (rho > 0.999; p < 0.001, each; Figure 4).
Table 2.
Comparative ATA titers toward the designated SB5 and ADL lots among anti-SB5 ATA+ and ATA– sera from patients with rheumatoid arthritis.
| SB5 |
ADL |
|||
|---|---|---|---|---|
| Sera type | Lot A | Lot B | Lot A | Lot B |
| ATA+, mean (SD) | 17.48 (14.24) | 16.88 (13.36) | 16.46 (13.19) | 16.60 (13.45) |
| ADL lot A, p value* for difference between lots | NS | NS | – | – |
| ADL lot B, p value for difference between lots | NS | NS | – | – |
| ATA–, mean (SD) | 0.81 (0.71) | 0.79 (0.70) | 0.71 (0.66) | 0.71 (0.69) |
| ADL lot A, p value* for difference between lots | <0.05 | <0.05 | – | – |
| ADL lot B, p value for difference between lots | <0.05 | <0.05 | – | – |
If normality was accepted (Shapiro-Wilk test), parametric methods were used for analysis; mean of difference between two lots and p value by paired t test were calculated.
ADL, reference adalimumab; ATA, antidrug antibody to adalimumab; NS, not significant.
Figure 3.
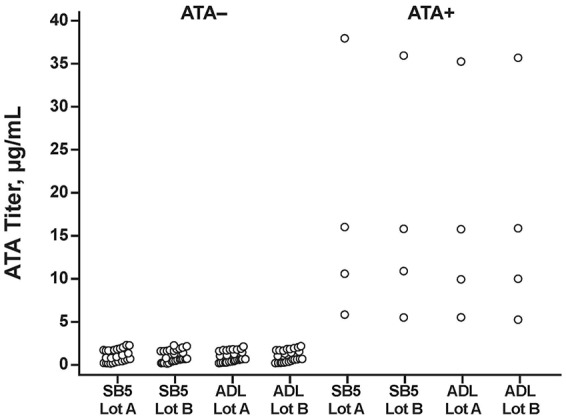
Comparative ATA titers toward the designated SB5 and ADL lots among anti-SB5 ATA+ and ATA– sera from patients with rheumatoid arthritis.
ADL, reference adalimumab; ATA, antidrug antibody to adalimumab; ATA+, positive for ATA; ATA–, negative for adalimumab.
Figure 4.
Correlation between ATA concentrations from patients with rheumatoid arthritis treated with SB5 or from negative controls, measured using the designated SB5 or ADL lots as the antigen for serum immune-reactivity. Correlation coefficient determined using Spearman correlation test.
ADL, reference adalimumab; ATA, antidrug antibody to adalimumab.
None of the 28 ATA– sera were reactive to either lot of SB5 or ADL. Mean (SD) concentrations for ATA– sera measured against SB5 lot A, SB5 lot B, ADL lot A, and ADL lot B were 0.81 (0.71), 0.79 (0.70), 0.71 (0.66), and 0.71 (0.69), respectively (Table 2; Figure 3). When analyzed using the Wilcoxon test, there was a significant difference between mean ATA titers measured against each lot of SB5 and ADL (Table 2), but the absolute difference between the means was ⩽0.10 μg/ml, which was within the negative background signal range of the assay.
Functional inhibition of TNF-α binding of SB5 and ADL
The five anti-ADL ATA+ sera from ADL-treated patients with IBD similarly neutralized the functional activity of SB5 and ADL; no functional inhibition was observed with the five ATA– sera (Figure 5).
Figure 5.
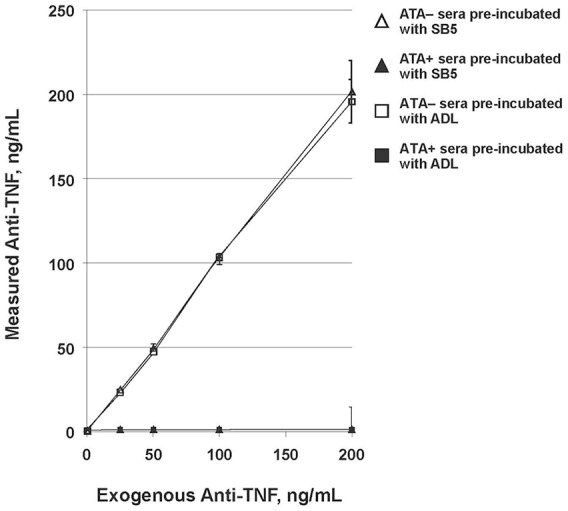
Neutralization assay of SB5 and ADL by anti-ADL ATA+ and ATA– sera.
ADL, reference adalimumab; ATA, antidrug antibody to adalimumab; ATA+, positive for ATA; ATA–, negative for ATA; TNF, tumor necrosis factor.
Cross-reactivity between SB2 and INF and between SB5 and ADL
The 11 ATI+ sera generated in INF-sensitized patients did not cross-react with SB5, whereas the five anti-ADL ATA+ sera generated in ADL-sensitized patients did recognize SB5 (Figure 6).
Figure 6.
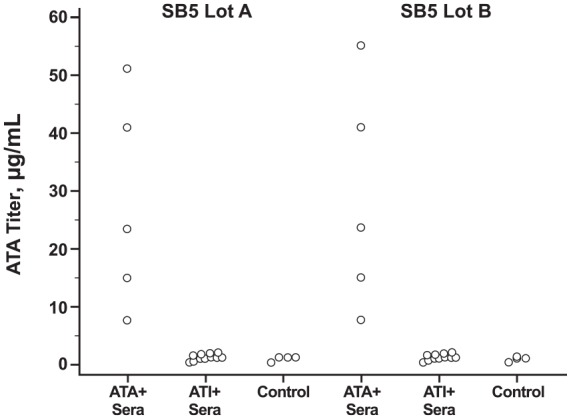
Cross-reactivity test of ATI and anti-ADL ATA from patients with inflammatory bowel disease toward SB5.
ATA, antidrug antibody to adalimumab; anti-ADL ATA+, positive for ATA; ATI, antidrug antibody to infliximab; ATI+, positive for ATI; control, antibody-negative sera from healthy adults.
Discussion
The availability of biosimilars for immune-mediated inflammatory diseases has improved access to treatment by reducing the economic burden.14 However, questions remain regarding use of biosimilars for the treatment of IBD as clinical trial data and real-world evidence are limited. Additionally, biosimilars were generally not tested in all disease states, and approval in IBD was provided based on extrapolation from clinical trials in other disease states.15,16
Our study included patients with IBD who had received ADL and patients with RA who had received SB5. The antibodies that developed in patients with IBD were cross-reactive with SB5, and antibodies that developed in patients with RA were cross-reactive with ADL. A strong correlation between ATA titers was detected between the ADL and SB5 lots. Although there was a slight but significant difference in ATA titer between one lot of SB5 and one lot of ADL (out of four comparisons), this difference likely has no functional importance because anti-ADL ATA+ similarly neutralized TNF-α binding in both SB5 and ADL lots. The low background signal that was generated in ATA– sera was slightly higher with SB5 than with ADL. However, all ATA– samples were well below the cut-off level of 2.3 μg/ml for ATA detection, and, functionally, there was no TNF inhibition of SB5 or ADL in ATA– sera. Taken together, the above results support shared immune-dominant epitopes between ADL and SB5 and thus a similar immunogenic profile. These results provide additional evidence that switching between ADL and SB5 would not affect immunogenicity rates, which is in agreement with previous studies of SB5 and other biosimilars in RA and psoriasis.17–20 However, more information on biological and physicochemical data of patients in the present study is needed to draw this conclusion.
The phase III trial for SB5 showed safety, efficacy, PK, and immunogenicity comparable with ADL in patients with moderate to severe RA.10 SB5 is approved by the European Commission for multiple indications, including IBD.7 Although the European Medicines Agency and European Commission have stated that harmful immunogenicity is unlikely after switching between a reference product and its biosimilar,21 the potential for increased immunogenicity has created initial concern about extrapolation with INF biosimilars in IBD.
Cross-reactivity, clinical trial, and real-world data for INF have shown switching from INF to SB2 and other biosimilars to be safe, with no evidence for increased risk of immunogenicity. One study in patients with IBD showed that antibodies developed against INF cross-reacted with CT-P13 and SB2, and that antibodies developed in patients treated only with CT-P13 cross-reacted with INF and SB2.22 Further, in patients switched from INF to CT-P13 who had developed ATI, a full cross-reaction pattern was shown for INF, SB2, and CT-P13.22 Other studies have also shown similar cross-immunogenicity results between INF, CT-P13, and SB2,12,13,16,23 and similar functional inhibition of TNF-α binding.12,16 The NOR-SWITCH study and its open-label extension showed that switching from INF to CT-P13 was not inferior to continued treatment with INF, and immunogenicity was not different between the two treatments.24,25 Although the NOR-SWITCH study enrolled a large number of patients with varying diagnoses, the number of patients with IBD was relatively small in the main trial (Crohn disease, n = 155; ulcerative colitis, n = 93), which limits the ability to generalize to a larger population. A randomized controlled trial in patients with Crohn disease showed similar immunogenicity between CT-P13 and INF in patients who switched between INF and CT-P13.26 Real-world data further support the safety of switching from INF to SB2 or CT-P13,27,28 reverse switching from CT-P13 to the reference product,29 and successive switching between INF and multiple INF biosimilars without a change in immunogenicity.30 Taken together, these results show that INF biosimilars and INF share immune-dominant epitopes.
Studies using commercial assays have demonstrated that anti-INF assays can detect SB2-specific antibodies, and that INF antibodies can inhibit SB2.31,32 Similarly, it has been shown that an anti-ADL assay can detect the ADL biosimilar ABP 501 as well as ADL. Biosimilars to ADL were approved after biosimilars to INF, and the pivotal trials for ADL biosimilars were conducted in patients with RA10,33 and psoriasis.20,34 In addition to pivotal trials, a study was conducted to determine cross-reactivity of antidrug antibodies between the reference ADL and ABP 501 in RA because of concerns regarding immunogenicity. A validated electrochemiluminescence-based assay and TNF-binding assay for neutralizing activity showed that ABP 501 and ADL are cross-reactive in RA.35 Although this was found to be the case in RA, it was unknown whether cross-reactivity would occur in IBD, raising possible concerns regarding immunogenicity. Our study is the first to show that antibodies to ADL in IBD similarly recognize and neutralize the functional activity of SB5 and ADL. We have also shown that not only do antibodies from ADL-treated patients with IBD recognize SB5, but antibodies from SB5-treated patients with RA similarly recognize ADL. Taken together, these results further support a shared immune-dominant epitope and immunogenic profile between SB5 and ADL. Combining biosimilars’ biological and physicochemical data, clinical trial results, and cross-reactivity data further supports that it is likely safe to switch from the reference product to its biosimilar, or from a biosimilar to the reference product. Our study also demonstrated that, as expected, anti-ADL ATA+ sera do cross-react with SB5; however, ATI+ sera do not cross-react with SB5, suggesting that SB5 does not share immunogenic epitopes with INF.
This study has a few limitations. The number of anti-SB5 ATA+ sera from patients with RA was small, limiting our ability to generalize to a larger population. In terms of cross-reactivity between ADL and SB5 in IBD, sera of the present study were from ADL-treated IBD patients. Further data looking at cross-reactivity between biosimilar and reference product from biosimilar-sensitized sera in patients with IBD will be useful to show interchangeability of immunogenicity between a biosimilar and its reference product. Further studies are needed to show comparable epitope localization between SB5 and ADL.
Conclusion
In sera from patients with either IBD or RA who had been previously exposed to ADL or SB5, respectively, antibodies to ADL and SB5 cross-react. These data support shared immune-dominant epitopes between ADL and SB5, and similar immunogenic profiles. ATI+ sera did not cross-react with SB5, suggesting a lack of shared immunogenic epitopes between INF and SB5.
Acknowledgments
Editorial support for development of this manuscript was provided by Krystina Neuman, at C4 MedSolutions, LLC (Yardley, PA, USA), a CHC Group company.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: This study and editorial support for development of this manuscript was supported by funding from Samsung Bioepis Co., Ltd.
Conflict of interest statement: J. Goncalves has received consulting fees from Astra Zeneca, Biogen, Novartis, Pfizer, Samsung Bioepis, and Sandoz.
G. Myung, M. Park, D. Jeong, and J. Ghil are employees of Samsung Bioepis Co., Ltd.
ORCID iD: Joao Goncalves  https://orcid.org/0000-0002-1245-3715
https://orcid.org/0000-0002-1245-3715
Contributor Information
Joao Goncalves, Faculty of Pharmacy at University of Lisbon, iMed Research Institute for Medicines, Av. Professor Gama Pinto, Lisbon 1649-003, Portugal.
Gihyun Myung, Samsung Bioepis Co., Ltd., Incheon, Republic of Korea.
MinJeong Park, Samsung Bioepis Co., Ltd., Incheon, Republic of Korea.
Deokyoon Jeong, Samsung Bioepis Co., Ltd., Incheon, Republic of Korea.
Jeehoon Ghil, Samsung Bioepis Co., Ltd., Incheon, Republic of Korea.
References
- 1. Dorner T, Strand V, Castaneda-Hernandez G, et al. The role of biosimilars in the treatment of rheumatic diseases. Ann Rheum Dis 2013; 72: 322–328. [DOI] [PubMed] [Google Scholar]
- 2. European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues (revision 1), https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active_en–0.pdf (2014, accessed 19 June 2019).
- 3. US Food and Drug Administration. Quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product: guidance for industry. Report, 2015. Silver Spring, MD: US Food and Drug Administration. [Google Scholar]
- 4. European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues (EMEA/CHMP/BMWP/42832/2005 Rev. 1), http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/01/WC500180219.pdf (2014, accessed 7 May 2019).
- 5. US Food and Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product: guidance for industry. Report, 2015. Silver Spring, MD: US Food and Drug Administration. [Google Scholar]
- 6. Danese S, Fiorino G, Raine T, et al. ECCO position statement on the use of biosimilars for inflammatory bowel disease-an update. J Crohns Colitis 2017; 11: 26–34. [DOI] [PubMed] [Google Scholar]
- 7. Imraldi (SB5). Summary of product characteristics. Delft, Netherlands: Samsung Bioepis NL B.V, 2017. [Google Scholar]
- 8. Lee N, Lee JJ, Yang H, et al. Evaluation of similar quality attribute characteristics in SB5 and reference product of adalimumab. MAbs 2019; 11: 129–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee JJ, Yang J, Lee C, et al. Demonstration of functional similarity of a biosimilar adalimumab SB5 to Humira®. Biologicals 2019; 58: 7–15. [DOI] [PubMed] [Google Scholar]
- 10. Weinblatt ME, Baranauskaite A, Niebrzydowski J, et al. Phase III randomized study of SB5, an adalimumab biosimilar, versus reference adalimumab in patients with moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol 2018; 70: 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buurman DJ, Blokzijl T, Festen EAM, et al. Quantitative comparison of the neutralizing capacity, immunogenicity and cross-reactivity of anti-TNF-α biologicals and an infliximab-biosimilar. PLoS One 2018; 13: e0208922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goncalves J, Santos M, Acurcio R, et al. Antigenic response to CT-P13 and infliximab originator in inflammatory bowel disease patients shows similar epitope recognition. Aliment Pharmacol Ther 2018; 48: 507–522. [DOI] [PubMed] [Google Scholar]
- 13. Ruiz-Arguello MB, Maguregui A, Ruiz Del Agua A, et al. Antibodies to infliximab in remicade-treated rheumatic patients show identical reactivity towards biosimilars. Ann Rheum Dis 2016; 75: 1693–1696. [DOI] [PubMed] [Google Scholar]
- 14. Singh SC, Bagnato KM. The economic implications of biosimilars. Am J Manag Care 2015; 21(Suppl. 16): s331–s340. [PubMed] [Google Scholar]
- 15. Argollo M, Fiorino G, Gilardi D, et al. Biosimilars of adalimumab in IBD: are we ready for that? Curr Pharm Des 2019; 25: 7–12. [DOI] [PubMed] [Google Scholar]
- 16. Ben-Horin S, Vande Casteele N, Schreiber S, et al. Biosimilars in inflammatory bowel disease: facts and fears of extrapolation. Clin Gastroenterol Hepatol 2016; 14: 1685–1696. [DOI] [PubMed] [Google Scholar]
- 17. Weinblatt ME, Baranauskaite A, Dokoupilova E, et al. Switching from reference adalimumab to SB5 (adalimumab biosimilar) in patients with rheumatoid arthritis: fifty-two-week phase III randomized study results. Arthritis Rheumatol 2018; 70: 832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen S, Pablos JL, Pavelka K, et al. An open-label extension study to demonstrate long-term safety and efficacy of ABP 501 in patients with rheumatoid arthritis. Arthritis Res Ther 2019; 21: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Papp K, Bachelez H, Costanzo A, et al. Clinical similarity of the biosimilar ABP 501 compared with adalimumab after single transition: long-term results from a randomized controlled, double-blind, 52-week, phase III trial in patients with moderate-to-severe plaque psoriasis. Br J Dermatol 2017; 177: 1562–1574. [DOI] [PubMed] [Google Scholar]
- 20. Blauvelt A, Lacour JP, Fowler JF, Jr, et al. Phase III randomized study of the proposed adalimumab biosimilar GP2017 in psoriasis: impact of multiple switches. Br J Dermatol 2018; 179: 623–631. [DOI] [PubMed] [Google Scholar]
- 21. European Medicines Agency and European Commission. Biosimilars in the EU: information guide for healthcare professionals. Report, 2017. London: European Medicines Agency and European Commission. [Google Scholar]
- 22. Fiorino G, Ruiz-Arguello MB, Maguregui A, et al. Full interchangeability in regard to immunogenicity between the infliximab reference biologic and biosimilars CT-P13 and SB2 in inflammatory bowel disease. Inflamm Bowel Dis 2018; 24: 601–606. [DOI] [PubMed] [Google Scholar]
- 23. Magro F, Rocha C, Vieira AI, et al. The new biosimilar of infliximab SB2 can be quantified by IFX-optimised therapeutic drug monitoring assays. Presented at the 13th Congress of the European Crohn’s and Colitis Organisation, 14–17 February 2018, Vienna, Austria. [Google Scholar]
- 24. Jorgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet 2017; 389: 2304–2316. [DOI] [PubMed] [Google Scholar]
- 25. Goll GL, Jorgensen KK, Sexton J, et al. Long-term efficacy and safety of biosimilar infliximab (CT-P13) after switching from originator infliximab: open-label extension of the NOR-SWITCH trial. J Intern Med 2019; 285: 653–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ye BD, Pesegova M, Alexeeva O, et al. Efficacy and safety of biosimilar CT-P13 compared with originator infliximab in patients with active Crohn’s disease: an international, randomised, double-blind, phase 3 non-inferiority study. Lancet 2019; 393: 1699–1707. [DOI] [PubMed] [Google Scholar]
- 27. Fischer S, Klenske E, Schmitt H, et al. Clinical outcomes and immunogenicity analysis over 6 months following a switch from originator infliximab (Remicade) to the biosimilar SB2 (Flixabi) in inflammatory bowel disease patients. Presented at the 13th Congress of the European Crohn’s and Colitis Organisation, 14–17 February 2018, Vienna, Austria. [Google Scholar]
- 28. Plevris N, Jones GR, Jenkinson PW, et al. Implementation of CT-P13 via a managed switch programme in Crohn’s disease: 12-month real-world outcomes. Dig Dis Sci 2019; 64: 1660–1667. [DOI] [PubMed] [Google Scholar]
- 29. Ilias A, Szanto K, Gonczi L, et al. Outcomes of patients with inflammatory bowel diseases switched from maintenance therapy with a biosimilar to Remicade. Clin Gastroenterol Hepatol. Epub ahead of print 8 January 2019. DOI: 10.1016/j.cgh.2018.1012.1036. [DOI] [PubMed] [Google Scholar]
- 30. Lauret A, Molto A, Abitbol V, et al. Effects of successive switches to different biosimilars infliximab on immunogenicity in chronic inflammatory diseases in daily clinical practice. Presented at the European Congress of Rheumatology 2019, 12–15 June 2019, Madrid, Spain. [DOI] [PubMed] [Google Scholar]
- 31. Magro F, Rocha C, Vieira AI, et al. The performance of Remicade®-optimized quantification assays in the assessment of Flixabi® levels. Therap Adv Gastroenterol 2018; 11: 1756284818796956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jentzer A, Berger AE, Labetoulle R, et al. Evaluation of infliximab and anti-infliximab LISA-TRACKER immunoassays for the therapeutic drug monitoring of SB2 infliximab biosimilar. Ther Drug Monit 2018; 40: 749–753. [DOI] [PubMed] [Google Scholar]
- 33. Cohen S, Genovese MC, Choy E, et al. Efficacy and safety of the biosimilar ABP 501 compared with adalimumab in patients with moderate to severe rheumatoid arthritis: a randomised, double-blind, phase III equivalence study. Ann Rheum Dis 2017; 76: 1679–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Papp K, Bachelez H, Costanzo A, et al. Clinical similarity of biosimilar ABP 501 to adalimumab in the treatment of patients with moderate to severe plaque psoriasis: a randomized, double-blind, multicenter, phase III study. J Am Acad Dermatol 2017; 76: 1093–1102. [DOI] [PubMed] [Google Scholar]
- 35. Miller J, Starcevic Manning M, Wala I, et al. Immunological cross-reactivity of anti-drug antibodies to adalimumab and ABP 501. Presented at the 13th Congress of the European Crohn’s and Colitis Organisation, 14–17 February 2018, Vienna, Austria. [Google Scholar]