Figure 1.
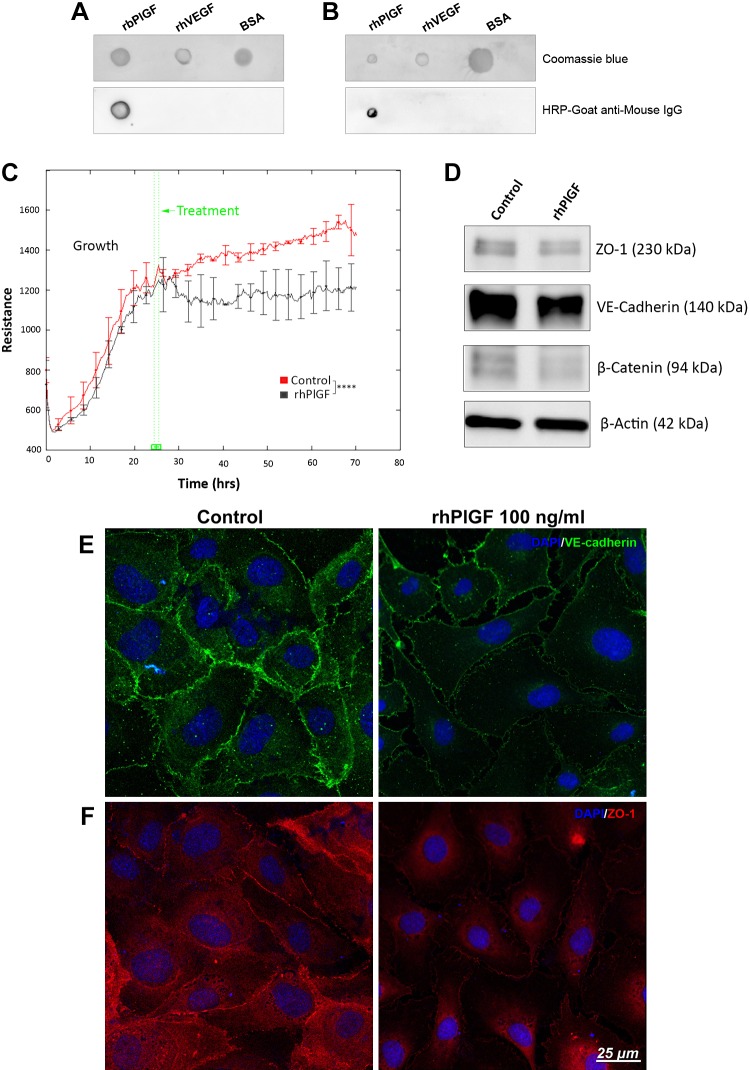
Recombinant PlGF has specific affinity with its mAb (PL5D11D4) and disrupts HREC barrier function. A, B) Dot immunoassay demonstrated the specific affinity of PlGF antibody (PL5D11D4) with rbPlGF (A) and rhPlGF (B). rhVEGF and BSA acted as negative controls. HRP, horseradish peroxidase. C) HRECs were cultured and grown with endothelial growth medium 2 (EGM-2) (with growth factors such as VEGF-A). After the cells grew to confluence, the culture medium was changed to endothelial cell growth basal medium 2 (EBM-2) (without growth factors), and PBS control and rhPlGF protein (100 ng/ml) were added to the culture medium and incubated for at least 2 d. TEER was monitored by an ECIS system at an AC frequency of 4 KHz in real time. TEER curves of HRECs that were treated with rhPlGF protein and PBS control. Error bars represent sd out of 4 duplicate samples. The experiments were repeated at least 3 times. D) WB result for VE-cadherin, β-catenin, and ZO-1. β-Actin was used as the protein loading control. E, F) Immunofluorescence staining results of tight junction ZO-1 and adhesion protein VE-cadherin. ****P < 0.0001.