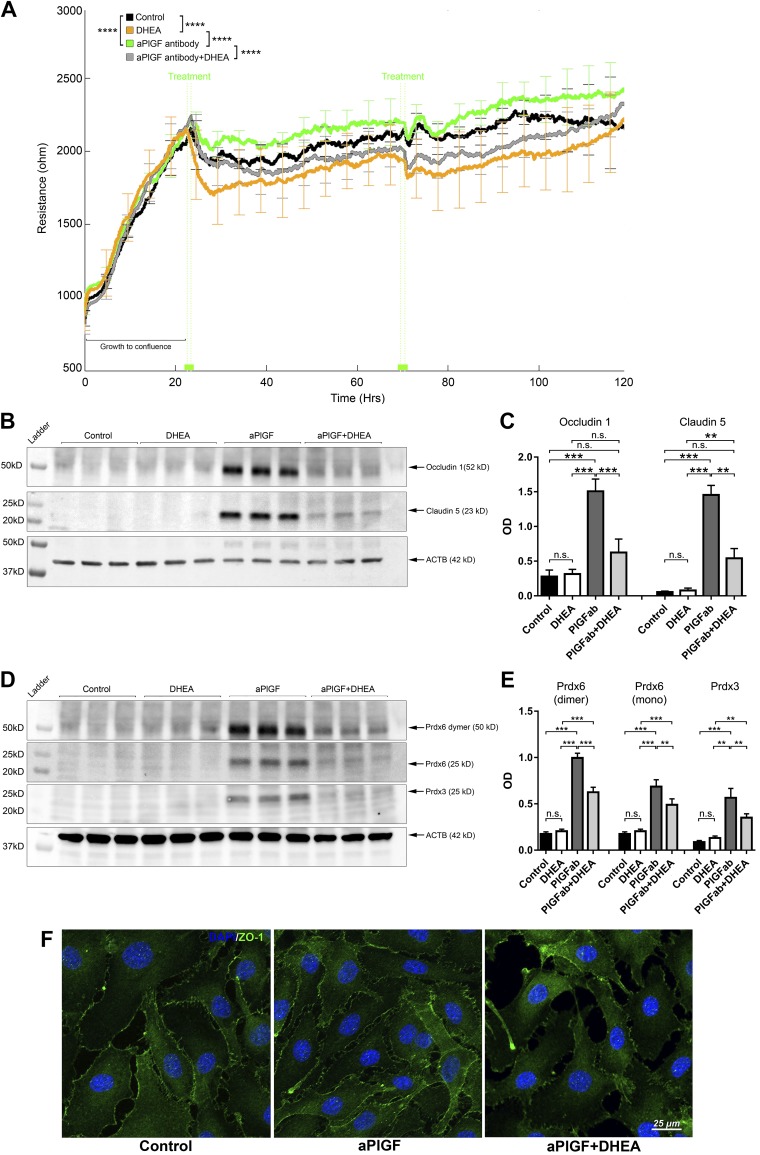
Figure 5.
G6PD inhibitor DHEA abrogates the effects of the PlGF antibody on HREC barrier function and PRDX3/6 protein expression. A) G6PD inhibitor DHEA abolished effects of PlGF antibody on HREC TEER, which was monitored by ECIS in real time. When the monolayer was formed, as indicated by the peak of the TEER values (mean ∼2200 Ω) at ∼24 h, treatment agents were added to the culture medium: PBS control, DHEA (25 µM), αPlGF (100 µg/ml), and DHEA + αPlGF. At ∼72 h, the same treatment schemes were reapplied. Four replicates were included for each treatment condition. Error bars represent sd out of 4 replicated samples. B, C) WB and densitometric quantification results for claudin-5 and occludin-1. DHEA abolished the effects of αPlGF on claudin-5 and occludin-1 protein expression. The treatment conditions were the same as those for the TEER experiments; however, the cells were grown on 6-well plates. For each sample, 50 µg proteins were loaded: β-actin (ACTB) was used as the protein loading control. D, E) WB and densitometric quantification results of PRDX3 and PRDX6. The G6PD inhibitor DHEA diminished the increased PRDX3 and PRDX6 protein expression by the PlGF antibody. F) Immunofluorescence staining of ZO-1. This illustrates increased ZO-1 allocation to the plasma membrane in the cell-cell interaction sites that were promoted by the PlGF antibody but prevented by the inhibitor DHEA. N.s., not significant. **P < 0.01, ***P < 0.001, ****P < 0.0001.