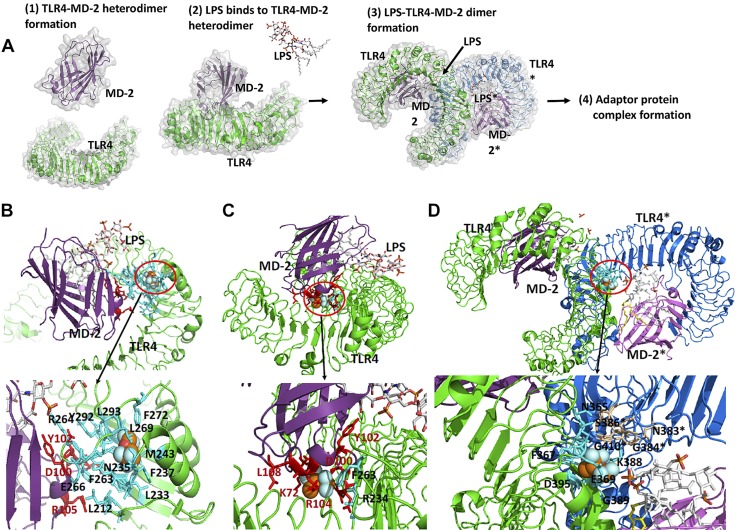
Figure 4.
The binding site of isoflurane on TLR4–MD-2 complex. A). Scheme of TLR4 activation by LPS binding. Prior to LPS binding, MD-2 forms a stable heterodimer with the extracellular domain of TLR4 (1). LPS binding induces dimerization of TLR4–MD-2 complex (2). This dimerization leads into a significant conformational change in the cytoplasmic domain, providing a new scaffold that allows the recruitment of specific adaptor proteins to form a post receptor signaling complex (3). Interaction with the downstream adaptors leads to NF-κB–mediated signaling. B, C). Isoflurane binding site at TLR4–MD-2 interface. Docked isoflurane near TLR4–Glu-262 (B), docked isoflurane near MD-2–Cys-105 (C). Nearby amino acids from isoflurane are shown in red for MD-2 and cyan for TLR4. D) Isoflurane binding site at dimer interface (TLR4-TLR4* interface). Isoflurane was docked near TLR4-Thr-413 residue. Nearby amino acids from isoflurane are shown in blue for TLR4 and in wheat for TLR4*. Blow-out pictures of isoflurane binding sites are shown.