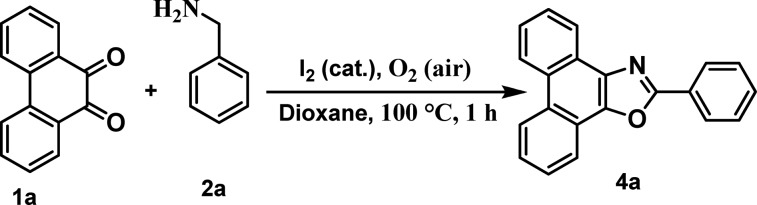Table 1. Development of C(sp3)-H-Functionalized 5-Annulationa.
| entry | catalyst | solventb | time (h) | yield (%)c |
|---|---|---|---|---|
| 1 | triflic acid | toluene | 15 | 48 |
| 2 | CSA | toluene | 15 | 57 |
| 3 | I2 | toluene | 2 | 79 |
| 4d | I2 | EtOH | 1.5 | 58 |
| 5 | I2 | EDC | 1.5 | 70 |
| 6 | I2 | DMF | 1.5 | 75 |
| 7 | I2 | DME | 1.5 | 78 |
| 8 | I2 | dioxane | 1 | 98 |
| 9d | I2 | dioxane | 6 | 95 |
| 10e | I2 | dioxane | 24 | 22 |
| 11f | I2 | dioxane | 12 | 79 |
| 12g | I2 | dioxane | 1 | 94 |
| 13 | dioxane | 24 | NDh |
Reaction conditions: 1a (0.5 mmol), 2a (0.5 mmol), catalyst (20 mol %).
Volume of solvent: 2 mL.
Yield of pure 4a after silica gel column chromatography using ethyl acetate in petroleum ether as an eluent.
Under reflux (∼80 °C for EtOH and ∼100 °C for dioxane).
Under argon.
Catalyst (15 mol %).
Catalyst (25 mol %).
Not detected.