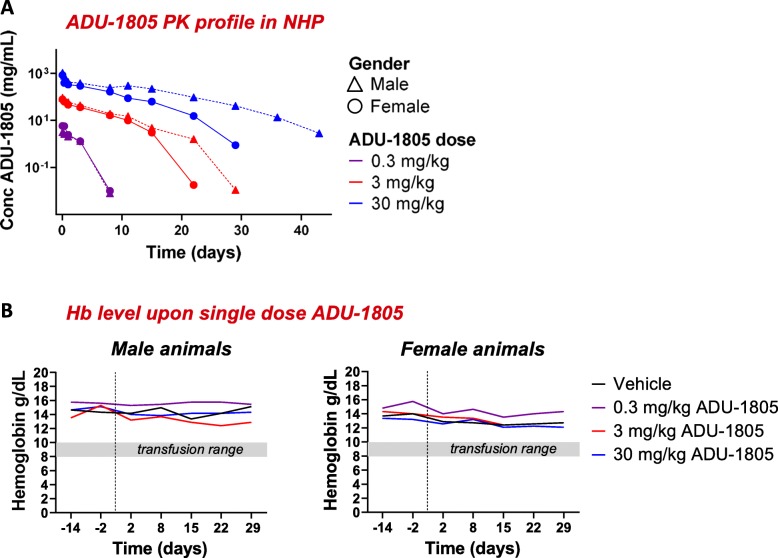
Fig. 6.
ADU-1805 can be safely administered intravenously in NHPs. a ADU-1805 single-dose pharmacokinetics profile in NHPs. Target-mediated drug disposition (TMDD) observed at the lowest dose. Dose proportional increase in exposure for the two higher dose levels (e.g. 3.0 mg/kg and 30 mg/kg). b ADU-1805 does not affect hemoglobin (Hb) levels in cynomolgus monkeys. Vertical dashed lines indicate infusion of monkeys on day 0. The shaded bar indicates the range of hemoglobin typically requiring a transfusion in humans [40]. (a, b: n = 6 animals)