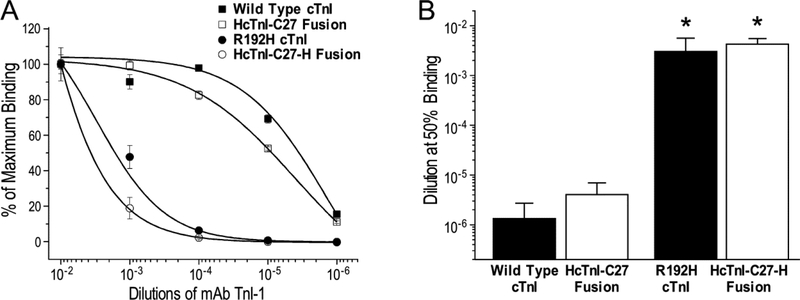
Figure 6: Similar affinities of mAb TnI-1 for HcTnI-C27 residing in SUMO fusion protein and in cardiac TnI.
The ELISA titration curves normalized to maximum binding (A) showed that mAb TnI-1 binds its epitope in Tx3-SUMO-HcTnI-C27 fusion protein and in wild type cardiac TnI (cTnI) with similar affinities as reflected by the mAb TnI-1 dilutions for 50% maximum binding (B), which were similarly decreased by the R to H single amino acid substitution in Tx3-SUMO-HcTnI-C27-H fusion protein and in situ in R192H RCM mutant cardiac TnI (*P < 0.005 compared with the wild type control in Student’s t test).