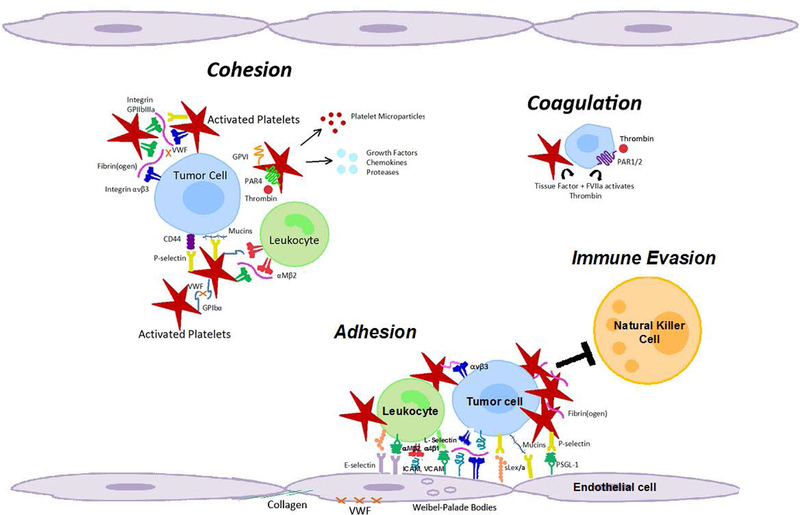
Figure 2. Molecular coordination between platelets and tumor cells supports metastasis from the blood stream.
As platelets become activated, they undergo a shape change, increase their adhesiveness, release granules and microparticles, and perpetuate cohesion of heteroaggregates containing tumor cells, platelets and leukocytes. Platelet granules contain growth factors, chemokines, and proteases. Cohesion: Circulating tumor cells can interact with activated platelets and leukocytes, and form heteroaggregates that support attachment to the endothelium and thereby contribute to metastasis. Coagulation: Platelet-initiated coagulation steps can activate receptors on tumor cells (e.g. PAR1/2) and promote release of tissue factor that further enhances procoagulant activity. Adhesion: Initial, transient adhesion involving selectins, mucins, CD44, and other sialyl Lewis X/A containing glycoconjugates is followed by firm attachment mediated by integrins and intercellular cell adhesion molecules. Immune evasion: Multivalent plasma proteins form intercellular bridges, and activated platelets and fibrin(ogen) protect tumor cells from natural killer cell lysis during hematogenous metastasis. Coincidently, tissue factor and FVIIa activate thrombin to cleave protease-activated receptors on platelets and tumor cells to promote invasion and metastasis. GP (Glycoprotein), PAR (Protease-activated receptor), ICAM (Intercellular adhesion molecule, ICAM-1), VCAM (Vascular cell adhesion molecule, VCAM-1), PSGL1 (P-selectin-glycoprotein ligand 1), sLex/a (Sialyl Lewis X/A antigens), VWF (Von Willebrand factor).