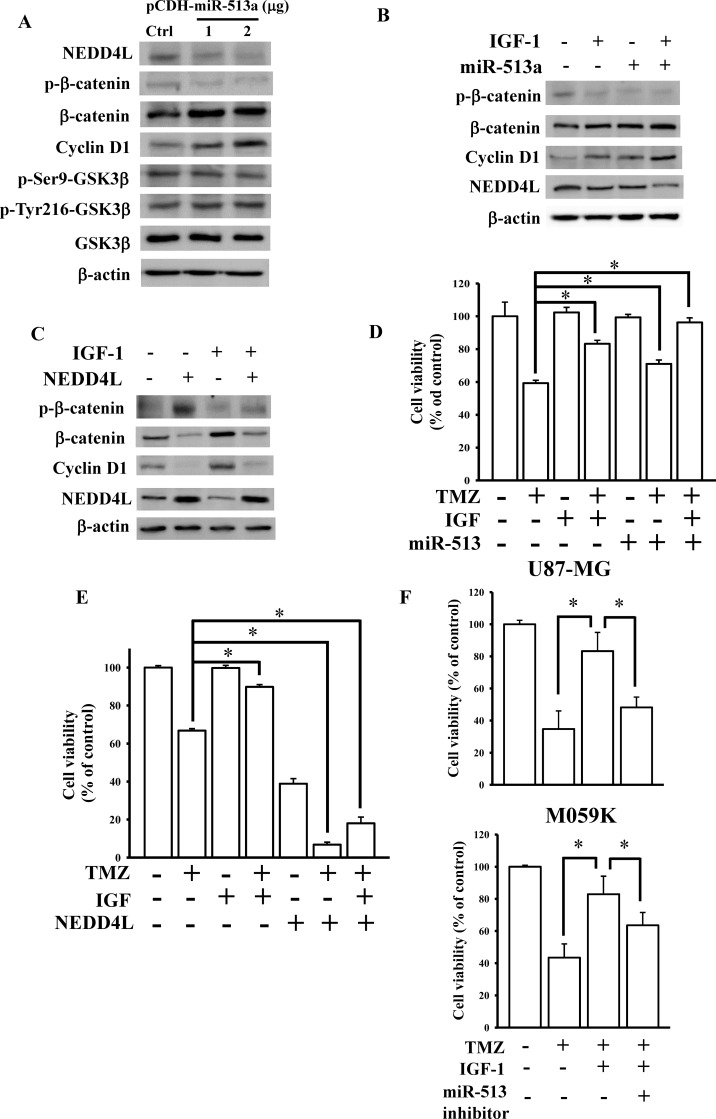
Fig 6. miR-513a-5p inhibited NEDD4L signaling in insulin-like growth factor (IGF)-1-desensitized glioma cells to temozolomide (TMZ).
(A) Effects of the overexpression of miR-513a-5p on downstream protein expressions of NEDD4L-inhibited WNT/β-catenin signaling. (B) Overexpression of miR-513a-5p enhanced the IGF-1-activated WNT/β-catenin pathway. (C) Overexpression of NEDD4L repressed the IGF-1-activated WNT/β-catenin pathway. After U87-MG cells were transfected with the indicated doses of the miR-513a-5p plasmid for 24 h, 1 μg of the miR-513a-5p plasmid combined with 200 ng/ml IGF-1 treatment for 48 h, or 2 μg NEDD4L plasmids combined with 200 ng/ml IGF-1 treatment for 48 h, WNT/β-catenin pathway downstream protein expressions were measured by an immunoblotting assay. Effects of miR-513a-5p (D) and NEDD4L (E) on IGF-1-reduced glioma sensitivity to TMZ. After U87-MG cells were transfected with 1 μg of the miR-513a-5p plasmid or 2 μg of the NEDD4L plasmid combined with 200 ng/ml IGF-1 and 200 μM TMZ treatment for 48 h, cell viability was measured by an MTT assay. Data are the mean ± SD of three independent experiments. * p<0.05. (F) Knockdown effects of miR-513a-5p on IGF-1-insensitized TMZ cytotoxicity. Cell viability was measured by a cell counting assay. Data are the mean ± SD of three independent experiments. * p<0.05.