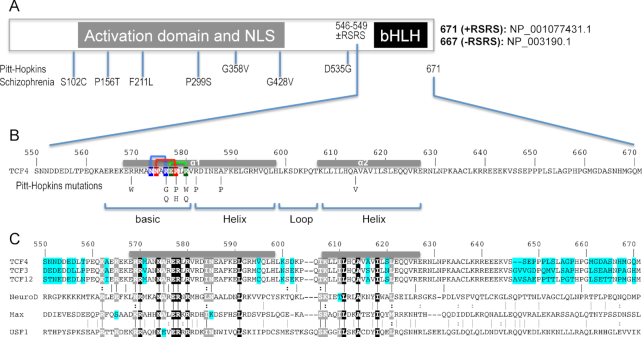
Figure 1.
Schematic of human TCF4 and sequence alignment of bHLH domains. (A) Human TCF4 transcripts potentially generate 18 isoforms with different N-termini (73), but all TCF4 isoforms contain the C-terminal bHLH DNA binding domain. In-frame alternative splicing increases the number of TCF4 isoforms. For example, alternative splicing at exon 18 of TCF4 leads to the presence or absence of two RS repeats––containing arginine (R) and serine (S)––immediately prior to the C-terminal bHLH domain. For the study described here, we use the residue numbering of +RSRS isoform (NP_001077431.1) for the bHLH domain. (B) Pitt-Hopkins mutations in bHLH that alter either the basic arginine residues at the protein–DNA interface or alanine residues that coordinate the dimerization. Three pairs of intra-molecular interactions exist in the major groove of DNA: N573•••R576 (blue), N574•••R578 (red) and E577•••R580 (green). (C) TCF3, TCF4, and TCF12 are Class I bHLH proteins, also called E-box binding proteins, and share high sequence identity within their bHLH domains, except for 7 positions (colored cyan). In contrast, other three representative proteins (NeuroD1, Max and USF1) used in the alignment shares only 9 invariant residues (white letters in black background) within the bHLH. White letters in grey background indicate conserved variation (R and K; I and L; T and S; L and M).