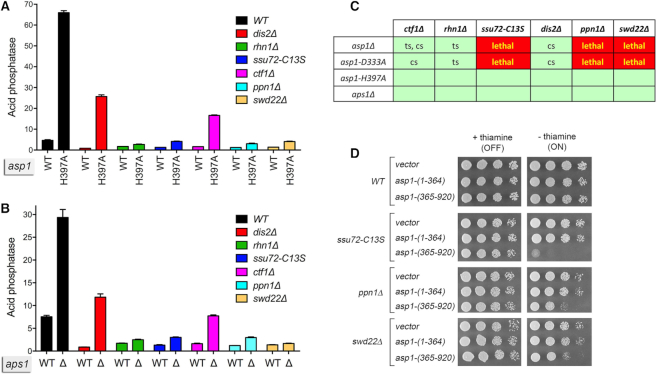
Figure 2.
Genetic and functional interactions between Asp1, Aps1, CPF subunits, and Rhn1. (A and B) De-repression of Pho1 expression by asp1-H397A and aps1Δ depends on CPF subunits and Rhn1. S. pombe strains bearing the indicated asp1 alleles (wild-type or H397A; panel A) or aps1 alleles (wild-type or aps1Δ; panel B) in combination with CPF subunit or Rhn1 mutations as specified were grown in liquid culture at 30°C and assayed for acid phosphatase activity. (C) Synthetic lethalities and growth defects. Synthetically lethal pairs of alleles are highlighted in red boxes. Viable double-mutants without a synthetic defect are indicated by plain green boxes. Viable double-mutants that displayed temperature-sensitive (ts) or cold-sensitive (cs) defects are annotated as such in their respective green boxes. (D) Asp1 pyrophosphatase domain overexpression inhibits growth of CPF subunit mutants. Wild-type, ssu72-C13S, ppn1Δ, and swd22Δ strains were transformed with plasmids overexpressing the Asp1 pyrophosphatase domain (aa 365–920) or the Asp1 kinase domain (aa 1–364) under the control of the thiamine-regulated nmt1 promoter (19). Control cells were transformed with the empty plasmid vector. After growth in PMG–Leu medium containing thiamine, serial dilutions were spotted on PMG–Leu medium that either contained 15 μM thiamine (nmt1 promoter OFF) or lacked thiamine (nmt1 promoter ON) and incubated at 30°C.