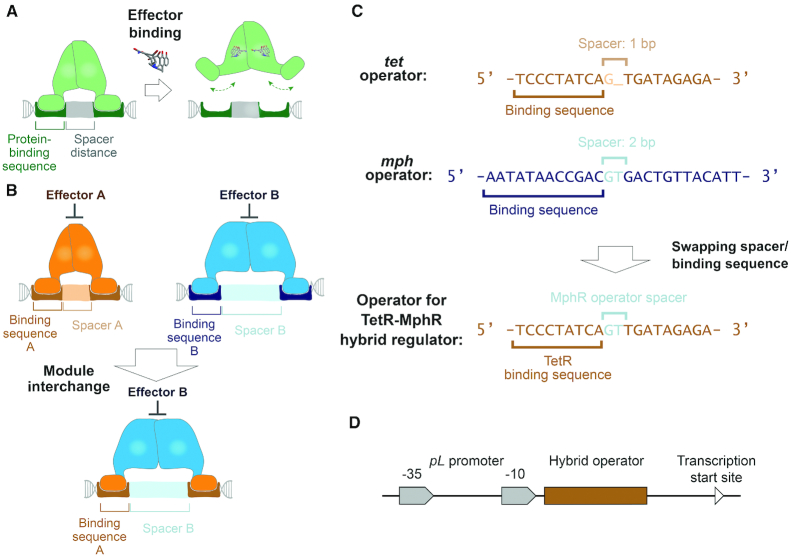
Figure 2.
The design principle of operators for hybrid regulators was established based on structural analyses of multiple TetR family members. (A) A conserved mechanism of action among regulators in the TetR family is illustrated in this panel. The ligand-binding module governs distance between the two DNA-binding domains that interact with the two protein-binding sequences to facilitate regulator-operating association. Thereby, a spacer with a specific length is required to align the two protein-binding DNA sequences to the two DNA-binding domains, such that regulator-operator binding is favorable. An effector molecule triggers conformational changes in the regulator, which alters the distance between the two DNA-binding domains and hampers interactions between the regulator and the operator. (B) If the DNA-binding domain of regulator B is replaced by homologous domains of regulator A, the hybrid regulator is expected to interact with protein-binding DNA sequences from operator A. However, since the DNA-binding domains’ interdistance is controlled by the ligand-binding module B, regulator-operator association requires the length of spacer to be the same as that in operator B. (C) Based on this rationale, we engineered an operator for the TetR-MphR hybrid regulator that is composed of a DNA-binding module from TetR and a ligand-binding module from MphR. This engineered operator contains two TetR-binding DNA sequences separated by a spacer from the MphR operator. (D) The engineered operator was inserted downstream of the −10 region of a pL promoter; binding of the regulator to the operator is expected to repress gene expression driven by the pL promoter.