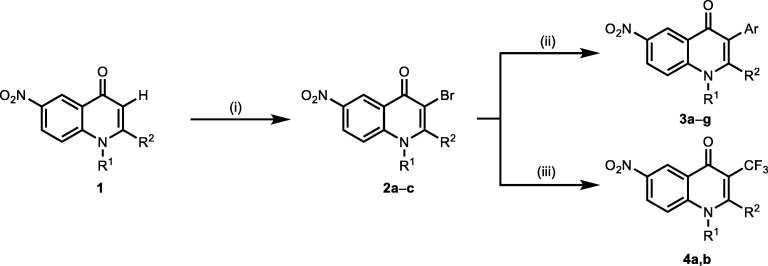
Scheme 3.
Modification strategies at the C–3 position in the 4–quinolinones. (Reagents and conditions: (i) 1.45 equiv. of NBS, CH3COOH, 20 °C, 1.5 h; (ii) 1.2 equiv. of aryl boronic acid, 0.1 equiv. of Pd(PPh3)4 10 equiv. K2CO3 in 5.5 mL of toluene with 1 mL of H2O and 1.5 mL of MeOH at 90 °C for 4 h; (iii) CF3COONa 4 equiv., CuI 8 equiv., DMA, 120 °C 6 h) [12]