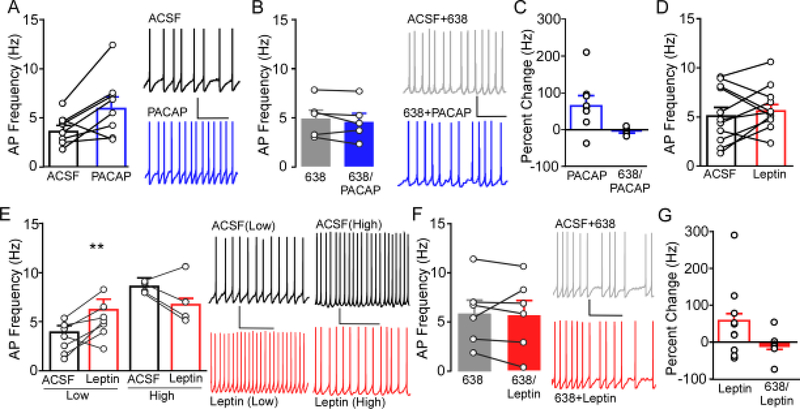
Figure 4.
Ex vivo slice electrophysiology was used to determine the role of PAC1R signaling on action potential (AP) frequency in the VMN. A) Application of 100nM PACAP (blue) in slice increases AP firing of neurons in the VMN (n= 8, N= 5) compared to baseline firing in ACSF (black). Representative trace of VMN AP firing (right) during bath application of ACSF (top) and following bath application of PACAP (bottom). * P<0.05 PACAP compared to ACSF. B) Prior and concomitant application of PACAP6–38 (6–38, gray filled) blocked the effects on AP firing induced by PACAP (6–38/PACAP, blue filled) (n=6, N=3). Representative traces of AP firing in the presence of PACAP6–38 (ACSF+6–38, top) and PACAP6–38 along with PACAP (6–38+PACAP, bottom). C) Percent change of firing produced by PACAP in the presence of ACSF (data from panel A) and in the presence of PACAP6–38 (data from panel B). D) Application of 100nM leptin (red) did not alter overall AP firing compared to baseline ACSF (n= 11, N= 5). D) Cells exhibiting a baseline AP frequency below the overall mean (Low) showed a significant increase in firing (left), whereas VMN cells showing a baseline AP frequency above the mean (High) showed a trend towards reduced firing (right). Representative traces (right) from Low and High cells in ACSF (top, black) and leptin (bottom, red). ** P<0.01 leptin compared to ACSF. F) Bath application of PACAP6–38 blocked the effects on AP firing induced by leptin (n= 6, N= 4). Representative traces of AP firing in PACAP6–38 + ACSF (top, gray filled) and PACAP6–38 + leptin (bottom, red filled). G) Percent change of firing produced by leptin in the presence of ACSF (data from panel D) and in the presence of PACAP6–38 (data from panel F).