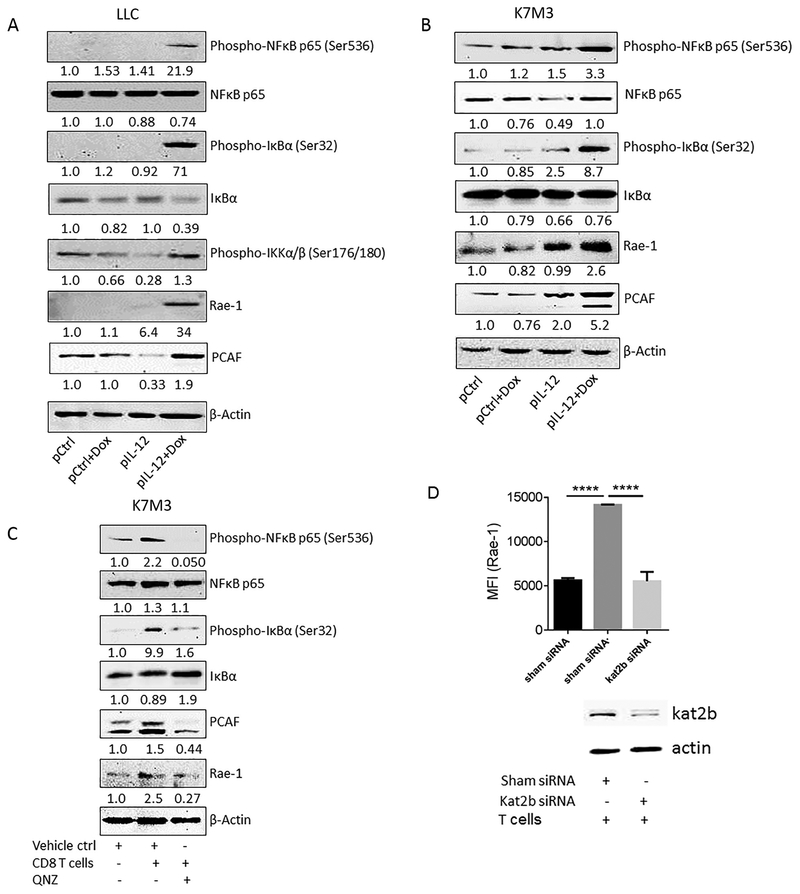
Fig. 3. NF-κB signaling and PCAF are induced in tumor cells upon CD8+ T cell engagement in vitro and in vivo.
(A, B) Mice (n=3/group) were treated twice (10 days apart) with one of the four indicated treatments: control, doxorubicin (Dox), IL-12–encoding DNA, or IL-12–encoding DNA plus doxorubicin. Tumors were collected on day 4 after the second treatment. Tumor samples were subjected to immunoblotting of phospho-NF-κB, NF-κB, phospho- IkBα, phospho- Ikkα/β, Rae-1, PCAF, and β-Actin. Values under each band indicate the density of protein expression. (C) K7M3 cells were pretreated with vehicle control or QNZ for 4 h, labeled with CFSE, and co-incubated with activated CD8+ T cells for 24 h. Tumor cells were isolated and subjected NF-κB and B and to immunoblotting of phospho-NF-κB, NF-κB, phospho-IkBα, IkBα, Rae-1, PCAF, and β-Actin. Values under each band indicate the density of protein expression. (D) K7M3 cells were transfected with control siRNA or PCAF (kat2b) siRNA, labeled with CFSE, and co-cultured with CD8+ T cells at the ratio of 1:1. The knockdown of PCAF was confirmed using immunoblotting, and Rae-1 levels on tumor cells were detected by using flow cytometry. MFI, mean fluorescence intensity. Bar graphs show means ± SEM. Results are representative of three repeated experiments. **** P<0.001.