Figure 2.
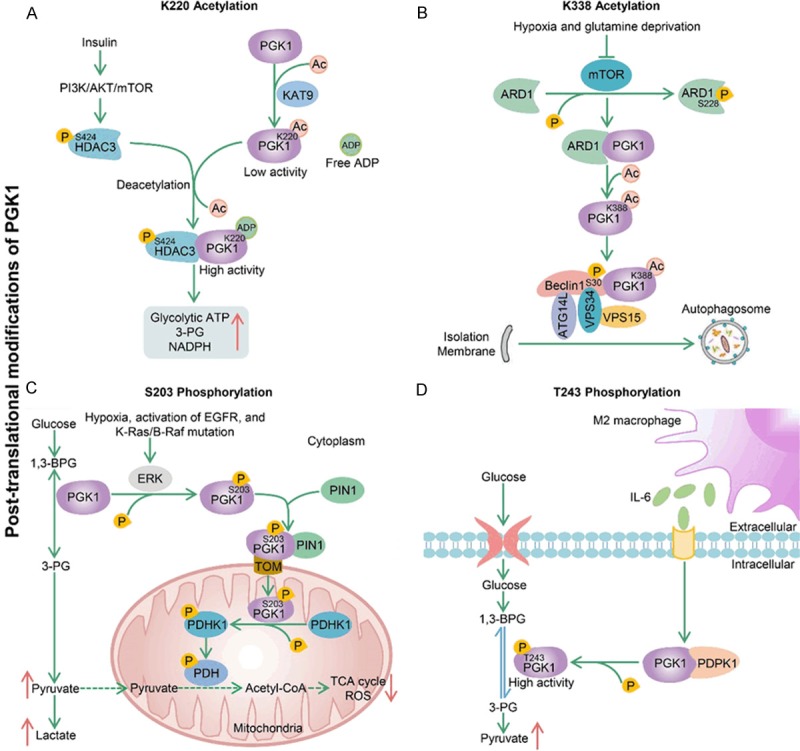
Several typical post-translational modifications of PGK1. A. KAT9 is a potential acetyltransferase of PGK1, which increases the K220 acetylation level and inhibits PGK1 enzyme activity by blocking its binding with substrate ADP. On the other hand, insulin-induced PI3K/AKT/mTOR pathway increases S424 phosphorylation of HDAC3, which promotes PGK1-HDAC3 interaction and PGK1 deacetylation at K220 to stimulate PGK1 activity. Finally, this HDAC3-dependent PGK1 deacetylation increases the production of glycolytic ATP, 3-PG and NADPH in cells. B. Hypoxia and glutamine deprivation inhibit mTOR-dependent ARD1 phosphorylation at S228, which enables ARD1 to interact with PGK1 for PGK1 acetylation. Next, the acetylated PGK1 binds to and phosphorylates Beclin1 at S30, which leads to activation of the VPS34-Beclin1-ATG14L complex for autophagosome formation. C. Signals (hypoxia, activation of EGFR, and K-Ras/B-Raf mutation) induce ERK-dependent phosphorylation of PGK1 at S203. Phosphorylated PGK1 is transported to mitochondria under the regulation of TOM complex and PIN1. Mitochondrial PGK1 functions as a protein kinase to phosphorylate and activate PDHK1, which further leads to phosphorylation and inactivation of PDH complex. This process results in the decrease of mitochondrial pyruvate metabolism and ROS generation and the enhancement of glycolysis in cytoplasm, and ultimately promotes GBM cell proliferation and tumorigenesis. D. M2-polarized macrophages secrete IL-6 to increase PDPK1-dependent PGK1 phosphorylation in GBM cells. This phosphorylation can reduce the 3-PG affinity of PGK1, which facilitates the equilibrium of the PGK1-catalyzed reaction toward glycolysis.
