Chronic kidney disease (CKD) is defined as abnormalities in the structure or function of the kidney that are present for more than 3 months and have implications for health. Inherited kidney diseases are a major cause of CKD and often lead to progressive CKD resulting in end-stage renal disease (ESRD). Cystic kidney diseases are common inherited causes of ESRD in both children and adults, accounting for 6%–12% of cases.S1,S2
Inherited forms of cystic kidney have been associated with dysfunction of the primary cilia.S3 These diseases are often termed renal ciliopathies and are part of a growing number of inherited diseases that include autosomal dominant polycystic kidney disease (ADPKD), autosomal recessive polycystic kidney disease (ARPKD),S4 tuberous sclerosis complex (TSC),S5 autosomal dominant tubulointerstitial kidney disease (ADTKD),S6 nephronophthisis-related ciliopathies (NPHP-RC),S7 Bardet-Biedl syndrome, Senior-Löken syndrome, Meckel Gruber syndrome, Joubert syndrome, and others.S8
ADPKD is the most common autosomal dominant inherited ciliopathy, accounting for 10% of all patients with ESRD requiring renal replacement therapy.S2 It is characterized by bilateral renal cysts, leading to enlarged kidneys and kidney failure. Extrarenal manifestations such as liver cysts, intracranial aneurysms, and mitral valve prolapse are also frequently noted. Most cases of ADPKD are caused by mutations in PKD1 (85%) and PKD2 (15%), although recently mutations in GANABS9 and DNAJB11S10 have been associated with similar phenotypes in genetically unresolved families with ADPKD. Clinically, ADPKD may manifest in either childhood or adulthood, and molecular genetic testing is becoming more important in this disease group, as it may reveal biallelic mutations/hypomorphic alleles in PKD1 or PKD2,S11,S12 and other genetic disorders, such as HNF1B, that may phenocopy ADPKD.S13
ARPKD may manifest in neonates as enlarged echogenic kidneys,S14 but it can also manifest in late childhood, adolescence, or adulthood.S15 It has an estimated incidenceS16 of 1:20,000 to 1:400,000 and is secondary to biallelic mutations in PKHD1.S17 Isolated or inbred populations, with their high rates of consanguinity, may have a much higher frequency of cases.S18 Compared with ADPKD, ARPKD presents much earlier and with greater disease severity, and up to 50% of affected neonates die shortly after birth from respiratory insufficiency secondary to pulmonary hypoplasia.S17 ARPKD is an important cause of ESRD in children, and molecular genetic testing allows testing of symptomatic and at-risk relatives and the consideration of preimplantation genetic diagnosis for future children.S19
Renal ciliopathies also include various forms of nephronophthisis,S7 which may be associated with multisystem phenotypes and diverse clinical features and considerable genetic heterogeneity. Nephronophthisis usually has an autosomal recessive mode of inheritance, and molecular genetics provides a more robust means of diagnosis compared with renal biopsy.S20
Molecular genetic analysis of individuals and families with cystic kidney disease is crucial in order to determine accurate diagnosis, prognosis, genetic counselling, and medical and educational management.S21–S23 However, genetic testing requires time and cost-effective approaches that will not overburden healthcare systems. Next generation sequencing (NGS, also called massively parallel sequencing) technologies are dramatically increasing sequencing capacity in routine clinical diagnosis, and speeding up genetic mutation identification. Gene panel approaches through parallel sequencing of targeted subsets of disease-associated genes as well as whole-exome and whole-genome sequencing are increasingly becoming part of routine clinical service for the investigation of inherited kidney disease.S24–S26 Specific to cystic kidney disease, various studies have applied NGS to deliver a disease-specific molecular diagnosis. Targeted NGS panels have been used extensively for analysis of ADPKD,1,S27 ARPKD,S17 and NPHP-RC.S28
In Oman, inherited kidney diseases are relatively common, leading to a significant healthcare burden.S29 Congenital malformations and genetic disorders are associated with 39% of perinatal deaths in hospitals.S29 A recent study showed that inherited kidney disease in Oman comprises just 5% of those reaching ESRD, reflecting a high mortality rate across newborns with inherited kidney disease.S30 However, to date, access to molecular genetic diagnostics is limited in Oman. We sought to establish an NGS platform for the molecular genetic diagnosis of cystic kidney disease in Oman that would allow for the first time a picture of the underlying molecular genetic causes of cystic kidney disease. In a pilot study of 53 patients with cystic kidney disease, we applied a diagnostic panel targeting 49 genes and achieved an overall molecular genetic diagnosis rate of 75%.
Methods
Results
The NGS panel (Supplementary Tables S1 and S2) showed a good capture yield and high sequencing quality with mean coverage depth of 875.3 ± 541 SD (Supplementary Table S3). An average of 2.6 million filtered reads were generated per sample, with 2.0 million of these aligned uniquely to the target region, leading to 76.5% average reads enrichment (65% to 83%) and hence indicating high sensitivity of the capture method used. On average, this panel provided 98.6%, 97.8%, 95.7%, and 89.3% base reads on target, with base coverage of 20×, 30×, 50×, and 100×, respectively (Supplementary Table S3). Sufficient coverage of all PKD1 exons was obtained through designing capture primers and enrichment technique (Supplementary Table S4).
The 53 patients included in this study were from different regions throughout Oman (Supplementary Figure S1) and included one prenatal (2%), 30 pediatric (from birth to 13 years; 57%), and 22 teenage/adult (>13 years; 42%) cases. The median age was 10 years (range: 0–63 years), and 29 (58%) were female. At the time of referral, 39 (74%) had a known family history of kidney disease.
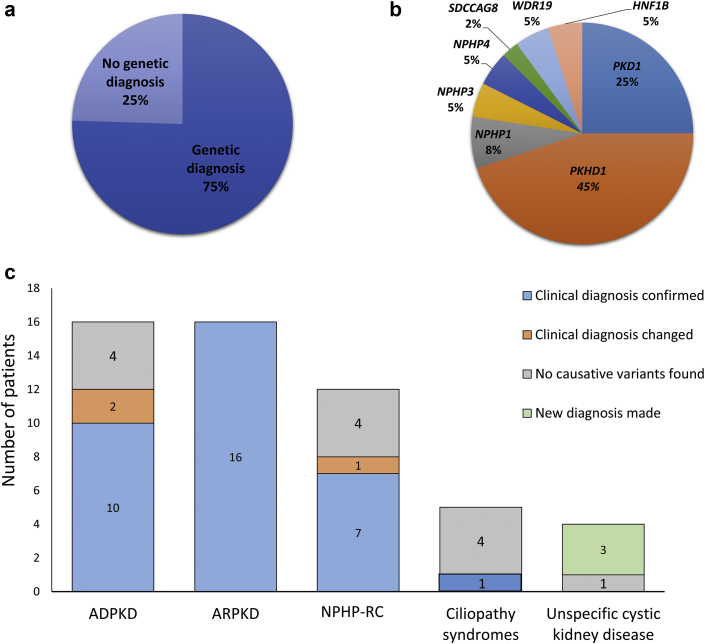
The clinical diagnosis of the patients included ADPKD (n = 16; 30%), ARPKD (n = 16; 30%), NPHP-RC (n = 12; 23%), ciliopathy syndromes (n = 5; 10%), and unspecified cystic kidney disease (n = 4; 7.5%). Clinical features and key family history details are shown in Supplementary Table S5. Molecular genetic investigations identified disease-causing variants in 40 of 53 (75%) patients (Figure 1a). Disease-causing variants were detected in PKD1, PKHD1, NPHP1, NPHP3, NPHP4, SDCCAG8, HNF1B, and WDR19 (Figure 1b). Upon evaluation, molecular genetic testing confirmed the clinical diagnosis of 33 (62%), changed the diagnosis in 3 (6%), and revealed a diagnosis in 3 patients (6%) with unspecified cystic kidney disease (Figure 1c). Overall, 12 (55%) variants were previously reported as disease causing, and 10 (46%) were novel (Table 11, 2, 3, 4, 5, 6, 7, 8, 9). Causative variants include 13 different single nucleotide variants (SNVs; 10 missense and 3 nonsense), 7 small insertions/deletions (INDELs; 5 deletions [≤20 base pairs] and 2 insertions), one large INDEL, and one whole-gene deletion (Table 1). According to the American College of Medical Genetics and Genomics, 17 variants were classified as pathogenic, 4 as likely pathogenic, and one as a variant of uncertain significance (Table 1).
Figure 1.
Molecular genetic diagnosis rate, genotype, and correlation with clinical phenotype. (a) Percentage of patients with an identified molecular genetic etiology of underlying cystic kidney disease. (b) Distribution of causative cystic kidney disease genes. (c) Comparison between suspected clinical diagnosis and molecular genetic diagnosis. ADPKD, autosomal dominant polycystic kidney disease; ARPKD, autosomal recessive polycystic kidney disease; NPHP-RC, nephronophthisis-related ciliopathies.
Table 1.
Molecular genetic findings
| Patient | Gender | Age at referral | Initial diagnosis | Gene | Transcript | Nucleotide change | Amino acid change | Type | Zygosity (inheritance) | MAF | ACMG classification | dbSNP ID | Family seg. confirmed | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | F | 47 yr | ADPKD | PKD1 | NM_001009944.2 | c.7428C>G | p.(Cys2476Trp) | SNV | Het (AD) | NA | Likely pathogenic | NA | Yes | Novel |
| P2 | F | 26 yr | ARPKD | PKHD1 | NM_138694.3 | c.9370C>T | p.(His3124Tyr) | SNV | Het (AR) | NA | Pathogenic | Yes | 4 | |
| NM_138694.3 | c.4870C>T | p.(Arg1624Trp) | SNV | Het (AR) | 1.81E-04 | Pathogenic | rs200391019 | 3 | ||||||
| P3 | M | 4 mo | ARPKD | PKHD1 | NM_138694.3 | c.107C>T | p.(Thr36Met) | SNV | Hom (AR) | 5.193E-04 | Pathogenic | rs137852944 | Yes | 5 |
| P5 | F | 12 yr | ARPKD | PKHD1 | NM_138694.3 | c.4870C>T | p.(Arg1624Trp) | SNV | Het (AR) | 1.812E-04 | Pathogenic | rs200391019 | No | 3 |
| NM_138694.3 | c.107C>T | p.(Thr36Met) | SNV | Het (AR) | 5.193E-04 | Pathogenic | rs137852944 | 5 | ||||||
| P6 | M | 2 yr | ARPKD | PKHD1 | NM_138694.3 | c.107C>T | p.(Thr36Met) | SNV | Hom (AR) | 5.193E-04 | Pathogenic | rs137852944 | No | 5 |
| P7 | M | 3 d | MKS | WDR19 | NM_025132.3 | c.2608G>A | p.(Asp870Asn) | SNV | Hom (AR) | 7.787E-04 | Uncertain significance | rs201963605 | Yes | No citation |
| P8 | F | 5 mo | ARPKD | PKHD1 | NM_138694.3 | c.107C>T | p.(Thr36Met) | SNV | Het (AR) | 5.193E-04 | Pathogenic | rs137852944 | Yes | 5 |
| NM_138694.3 | c.406A>G | p.(Thr136Ala) | SNV | Het (AR) | NA | Pathogenic | Novel | |||||||
| P9 | M | 16 yr | ARPKD | PKHD1 | NM_138694.3 | c.107C>T | p.(Thr36Met) | SNV | Hom (AR) | 5.193E-04 | Pathogenic | rs137852944 | No | 5 |
| P11 | F | 19 yr | ARPKD | PKHD1 | NM_138694.3 | c.107C>T | p.(Thr36Met) | SNV | Hom (AR) | 5.193E-04 | Pathogenic | rs137852944 | No | 5 |
| P14 | F | 1 yr | NPHP | NPHP3 | NM_153240.4 | c.3448C>T | p.(Gln1150*) | SNV | Hom (AR) | NA | Pathogenic | Yes | Novel | |
| P16 | F | 2 mo | ARPKD | PKHD1 | NM_138694.3 | c.107C>T | p.(Thr36Met) | SNV | Hom (AR) | 5.193E-04 | Pathogenic | rs137852944 | No | 5 |
| P18 | M | 41 yr | ADPKD | PKD1 | NM_001009944.2 | c.12604_12631delGGCCGGCTGGGGACAAGGTGTGAGCCTG | p.(Gly4202fs*146) | INDEL | Het (AD) | NA | Pathogenic | Yes | 1 | |
| P20 | F | 43 yr | ADPKD | PKD1 | NM_001009944.2 | c.5014_5015delAG | p.(Arg1672fs*97) | INDEL | Het (AD) | NA | Pathogenic | Yes | 1 | |
| P21 | U/A | Fetus | UCKD | NPHP3 | NM_153240.4 | c.2529delA | p.(Tyr844Thrfs*5) | INDEL | Hom (AR) | NA | Pathogenic | Yes | Novel | |
| P22 | M | 3 yr | ARPKD | PKHD1 | NM_138694.3 | c.406A>G | p.(Thr136Ala) | SNV | Hom (AR) | NA | Pathogenic | Yes | Novel | |
| P23 | M | 12 yr | NPHP | NPHP4 | NM_015102.3 | c.3784A>T | p.(Arg1262*) | SNV | Hom (AR) | NA | Pathogenic | No | Novel | |
| P24 | F | 8 yr | NPHP | SDCCAG8 | NM_006642.3 | c.1420delG | p.(Glu474fs*493) | INDEL | Hom (AR) | 1.65E-05 | Pathogenic | rs397515335 | Yes | 6 |
| P25 | F | 2 mo | ARPKD | PKHD1 | NM_138694.3 | c.107C>T | p.(Thr36Met) | SNV | Hom (AR) | 5.193E-04 | Pathogenic | rs137852944 | Yes | 5 |
| P26 | F | 17 yr | ARPKD | PKHD1 | NM_138694.3 | c.107C>T | p.(Thr36Met) | SNV | Hom (AR) | 5.193E-04 | Pathogenic | rs137852944 | Yes | 5 |
| P27 | M | 20 yr | ARPKD | PKHD1 | NM_138694.3 | c.107C>T | p.(Thr36Met) | SNV | Het (AR) | 5.193E-04 | Pathogenic | rs137852944 | Yes | 5 |
| NM_138694.3 | c.406A>G | p.(Thr136Ala) | SNV | Het (AR) | NA | Pathogenic | Novel | |||||||
| P28 | F | 28 yr | ARPKD | PKHD1 | NM_138694.3 | c.107C>T | p.(Thr36Met) | SNV | Het (AR) | 5.193E-04 | Pathogenic | rs137852944 | Yes | 5 |
| NM_138694.3 | c.4870C>T | p.(Arg1624Trp) | SNV | Het (AR) | 1.81E-04 | Pathogenic | rs200391019 | 3 | ||||||
| P29 | F | 1 yr | ARPKD | PKHD1 | NM_138694.3 | c.107C>T | p.(Thr36Met) | SNV | Het (AR) | 5.193E-04 | Pathogenic | rs137852944 | Yes | 5 |
| NM_138694.3 | c.4870C>T | p.(Arg1624Trp) | SNV | Het (AR) | 1.812E-04 | Pathogenic | rs200391019 | 3 | ||||||
| P32 | M | 40 yr | ADPKD | PKD1 | NM_001009944.2 | c.6264dupG | p.(Arg2089Alafs*19) | INDEL | Het (AD) | NA | Pathogenic | Yes | Novel | |
| P33 | M | 31 yr | ADPKD | PKD1 | NM_001009944. | c.12301delC | p.(Leu4101Trpfs*97) | INDEL | Het (AD) | NA | Pathogenic | Yes | Novel | |
| P34 | F | 55 yr | ADPKD | PKD1 | NM_001009944.2 | c.12604_12631delGGCCGGCTGGGGACAAGGTGTGAGCCTG | p.(Gly4202fs*146) | INDEL | Het (AD) | NA | Pathogenic | Yes | 1 | |
| P37 | M | 53 yr | ADPKD | PKD1 | NM_001009944.2 | c.9340C>T | p.(Gln3114*) | SNV | Het (AD) | NA | Pathogenic | Yes | Novel | |
| P38 | F | 2 yr | ARPKD | PKHD1 | NM_138694.3 | c.107C>T | p.(Thr36Met) | SNV | Hom (AR) | 5.193E-04 | Pathogenic | rs137852944 | No | 5 |
| P39 | F | 13 yr | NPHP | HNF1B | NM_000458.2 | c.443C>T | p.(Ser148Leu) | SNV | Het (de novo) | NA | Likely pathogenic | rs121918674 | No | 7 |
| P40 | M | 12 yr | NPHP | NPHP4 | NM_015102.3 | c.673G>T | p.(Gly225Cys) | SNV | Hom (AR) | 1.15E-05 | Likely pathogenic | rs540402276 | No | No citation |
| P42 | F | 7 yr | ADPKD | PKD1 | NM_001009944.2 | c.7421dupG | p.(Ser2475Leufs*26) | INDEL | Het (de novo) | NA | Pathogenic | No | Novel | |
| P43 | M | 47 yr | ADPKD | PKD1 | NM_001009944.2 | c.2711_2712delAG | p.(Glu904Glyfs*196) | INDEL | Het (AD) | NA | Pathogenic | Yes | Novel | |
| P44 | M | 5 mo | ARPKD | PKHD1 | NM_138694.3 | c.107C>T | p.(Thr36Met) | SNV | Hom (AR) | 5.193E-04 | Pathogenic | rs137852944 | Yes | 5 |
| P45 | M | 44 yr | ADPKD | PKD1 | NM_138694.3 | c.6264dupG | p.(Arg2089Alafs*19) | INDEL | Het (AD) | NA | Pathogenic | Yes | Novel | |
| P46 | F | 10 yr | NPHP | NPHP1 | NM_001009944.2 | NPHP1 deletion | WGD | Hom (AR) | 0.0024 | Pathogenic | No | 8 | ||
| P48 | M | 13 yr | UCKD | NPHP1 | NM_025132.3 | NPHP1 deletion | WGD | Hom (AR) | 0.0024 | Pathogenic | No | 8 | ||
| P49 | F | 2 mo | NPHP | WDR19 | NM_000458.2 | c.3533G>A | p.(Arg1178Gln) | SNV | Hom (AR) | 1.304E-04 | Likely pathogenic | rs79436363 | No | 2 |
| P50 | F | 11 yr | NPHP | NPHP1 | NM_138694.3 | NPHP1 deletion | WGD | Hom (AR) | 0.0024 | Pathogenic | No | 8 | ||
| P51 | M | 8 yr | ADPKD | HNF1B | NM_138694.3 | c.494G>A | p.(Arg165His) | SNV | Het (de novo) | NA | Pathogenic | rs121918675 | Yes | 9 |
| P52 | M | 2 d | UCKD | PKHD1 | NM_138694.3 | c.107C>T | p.(Thr36Met) | SNV | Hom (AR) | 5.193E-04 | Pathogenic | rs137852944 | Yes | 5 |
| P53 | M | 23 yr | ADPKD | PKHD1 | NM_138694.3 | c.107C>T | p.(Thr36Met) | SNV | Het (AR) | 5.193E-04 | Pathogenic | rs137852944 | No | 5 |
| NM_138694.3 | c.406A>G | p.(Thr136Ala) | SNV | Het (AR) | NA | Pathogenic | Novel | |||||||
ACMG, American College of Medical Genetics and Genomics; AD, autosomal dominant; ADPKD, autosomal dominant kidney disease; AR, autosomal recessive; ARPKD, autosomal recessive kidney disease; BBS, Bardet Biedl syndrome; d, day; dbSNP, single-nucleotide polymorphism database; F, female; Het, heterozygous; Hom, homozygous; INDEL, insertion/deletion; JBTS, Joubert syndrome; MAF, minor allele frequency; M, male; MKS, Meckel Gruber syndrome; mo, months; NA, not applicable; NPHP, nephronophthisis; seg, segregation; SNV, single-nucleotide variant; U/A, unavailable; UCKD, unspecific cystic kidney disease; WGD, whole-gene deletion.
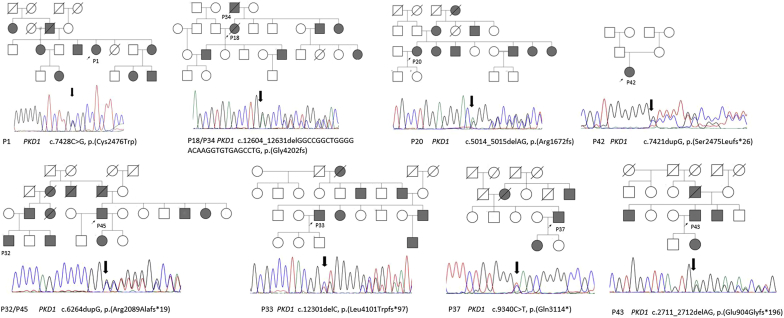
The majority of patients referred for ADPKD were teenagers/adults (88%; 14 of 16). Genetic testing identified 8 different PKD1 pathogenic variants, including 1 novel missense, 1 novel nonsense alteration, 2 small deletions, 2 novel small insertions, and 1 large INDEL (Table 1 and Figure 2).
Figure 2.
Pathogenic PKD1 variants detected in Omani autosomal dominant polycystic kidney disease patients. Pedigree diagrams showing family structure and chromatograms showing PKD1 pathogenic variants that were detected and Sanger confirmed in patients (P) with autosomal dominant polycystic kidney disease. Squares specify males; circles specify females. Arrow points to proband; filled squares and circles specify affected individuals in the family.
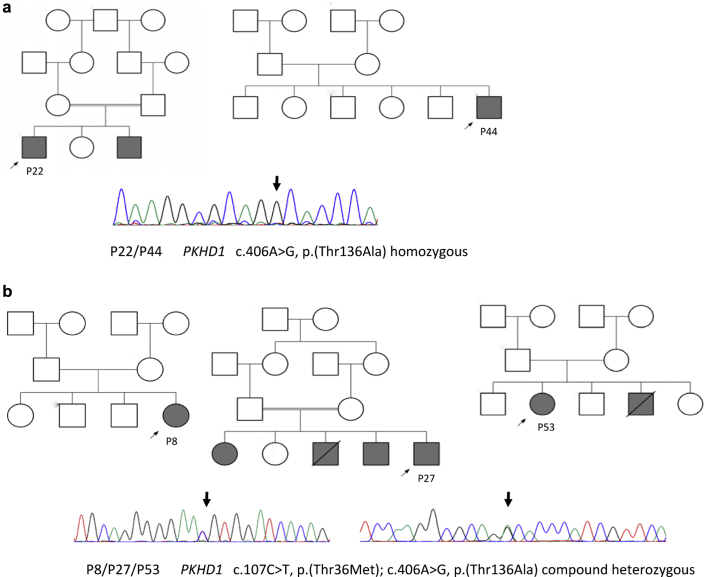
A molecular genetic etiology of ARPKD was obtained in a total of 18 unrelated patients, in which 4 PKHD1 different missense variants were detected. These variants were c.107C>T, p.(Thr36Met); c.406A>G, p.(Thr136Ala); c.4870C>T, p.(Arg1624Trp), and c.9370C>T, p.(His3124Tyr) (Table 1). The c.107C>T, p.(Thr36Met) PKHD1 variant was identified homozygously in 10 patients and compound heterozygously in 4 other patients (Table 1). The p.(Thr136Ala) allele (Figure 3) has not been reported previously or described in any databases. However, mutation evaluation algorithms considered this variation pathogenic. As there was only a limited number of PKDH1 variants detected in unrelated Omani individuals, this suggested the possibility of founder mutations within PKHD1.
Figure 3.
Pathogenic PKHD1 variants detected in Omani autosomal recessive polycystic kidney disease patients. (a) Family pedigree of patients (P) P22 and P44 and Sanger sequencing chromatogram showing novel PKHD1 pathogenic homozygous missense variant c.406A>G p.(T136A). (b) Family pedigree of patients P8, P27, and P53 and Sanger sequencing in which variant c.406A>G p.(T136A) was detected in compound heterozygous with c.107C>T, p.(T36M). Squares indicate males; circles indicate females. Arrows point to the proband; filled squares and circles indicate affected individuals in the family.
A total of 9 patients were found to carry causative variants associated with NPHP-RC in NPHP1, NPHP3, NPHP4, SDCCAG8, and WDR19 (Table 1). Copy number variations were detected in 3 of the patients (P46, P48, and P50), in which a homozygous deletion of ∼862 kb in size that contains 16 genes, including 3 OMIM genes—NPHP1 (OMIM: 607100), RGPD6 (OMIM: 612709), and MALL (OMIM: 602022)—was validated by comparative genomic hybridization array (Supplementary Figure S2).
Two novel pathogenic variants were detected in the NPHP3 gene. A homozygous nonsense variant p.(Gln1150*) was identified in a 1-year-old female (P14) who presented with hypertension, cystic kidney disease, liver fibrosis, splenomegaly, and ESRD (Table 1). In a second consanguineous family (P21), we identified a homozygous 1-bp deletion in c.2529delA in exon 18 of the NPHP3 gene in a fetal sample, resulting in frameshift and premature termination of p.(Tyr844Thrfs*5). This family has a history of 2 fetal deaths with features of oligohydramnios, and antenatal scan showed bilateral enlarged kidneys with loss of corticomedullary differentiation and lung hypoplasia (Supplementary Table S5).
A homozygous missense p.(Gly225Cys) and nonsense p.(Arg1262*) variant were detected in NPHP4 in probands P40 and P23, respectively, both with a clinical diagnosis of NPHP (Supplementary Table S5). The p.(Gly225Cys) variant (rs540402276) is reported in the ExAC database only once in an African population in its heterozygous state with a very low minor allele frequency, whereas p.(Arg1262*) is a novel nonsense variant.
A homozygous frameshift deletion p.(Glu474fs*20) in the SDCCAG8 gene was identified in P24 with a clinical diagnosis of NPHP, reaching ESRD at the age of 8 years and extra renal features of retinitis pigmentosa (Supplementary Table S5). A 2-month-old patient (P49) with renal impairment, dilated bile ducts, and bilateral echogenic kidneys was found to carry a known homozygous missense variant p.(Arg1178Gln) located in exon 31 of the WDR19 gene (rs79436363; Table 1) that had previously been reported in a patient with Senior-Loken syndrome-8.2
The NGS panel was able to provide a new molecular diagnosis for some patients with a de novo mode of inheritance. For example, in a 10-year-old male (P51) with CKD secondary to bilateral echogenic kidneys mimicking ADPKD but without a family history of disease, a heterozygous pathogenic SNV p.(Arg165His) was identified in HNF1B (Table 1). Segregation analysis of parents and unaffected siblings revealed this to be a likely de novo mutation. Another assumed de novo HNF1B missense variant p.(Ser148Leu) was identified in a 13-year-old female (P39) who had antenatal polycystic kidneys and no family history of disease (Table 1). A molecular genetic diagnosis was found in one patient (P7) with a multisystem ciliopathy suggestive of Meckel Gruber syndrome, including dysmorphic features, encephalocele, and polydactyl. In this patient, a homozygous missense variant p.(Asp870Asn) (rs201963605) in the WDR19 gene was detected that co-segregated with the other affected sibling with a similar phenotype.
The gene panel failed to identify a molecular genetic diagnosis in 13 patients, including 4 patients with an ADPKD-like phenotype (P4, P35, P36, and P47), 5 patients with an NPHP-like phenotype (P10, P15, P17, P30, and P31), and 4 with a more complex multisystem ciliopathy phenotype including Meckel Gruber syndrome (P12 and P41), Joubert syndrome (P13), and Bardet-Biedl syndrome (P19).
Discussion
Employing NGS diagnostic panels for the high-throughput detection of disease-causative variants through interrogation of multiple genes simultaneously has become a common approach in the genetic testing of inherited kidney disease. The present study represents the first comprehensive genetic analysis of inherited cystic kidney diseases and renal ciliopathies in patients from Oman using a customized NGS panel. Our results demonstrate the efficiency of the design and application of this targeted panel for genetic diagnosis of patients with different phenotypes.
Generally speaking, the diagnostic yield of NGS panels greatly depends on the patient population selected and the variant calling threshold. We have shown that an NGS panel consisting of 49 cystic kidney disease–associated genes applied to 53 proband patients was capable of resolving 75%, consistent with the reported diagnostic yield of other targeted NGS studies.S31 Our high rate of detection may be explained by clear phenotypic characterization of patients and detailed family history. As a result, the clinical diagnosis was confirmed by molecular testing in 33 (62%) patients. The molecular diagnostic results enabled a change in clinical diagnosis in 3 patients (resolving the overlapping clinical phenotypes of NPHP/ARPKD and ADPKD/renal cysts and diabetes syndrome) and a precise diagnosis in 3 patients who had an unclear or atypical cystic kidney disease phenotype (Table 1).
Defining the genetic etiology of disease is fundamental in terms of medical intervention, disease management, and future family planning. Overall, a genetic etiology was obtained in 73% of pediatric patients, in which ARPKD (37%) and NPHP-RC (27%) were the most prevalent disease. In contrast, genetic diagnosis was achieved in 77% of adults, most of whom had either ADPKD (41%) or ARPKD (32%). Similar to other studies, no significant difference was evident among pediatric and adult patients, in terms of genetic diagnostic rate.S23
Among the 49 panel genes, only 8 contributed to our diagnostic yield. These were PKHD1 (45%), PKD1 (25%), NPHP1 (8%), NPHP3 (5%), NPHP4 (5%), WDR19 (5%), HNF1B (5%), and SDCCAG8 (2%). In total, 22 different disease-causative variants were identified in 40 patients, which include 10 missense, 3 nonsense, 5 small deletion, 3 small insertions, one large INDEL, and a whole-gene deletion affecting NPHP1 (Supplementary Figure S2). Of those, 10 (46%) variants were novel findings in the genes PKD1 (n = 6), PKHD1 (n = 1), NPHP3 (n = 2), and NPHP4 (n = 1). Homozygous variants/deletions were detected in more than half our patients (23 of 40), in keeping with the known consanguinity. The custom of consanguineous marriages is strongly adhered to in the Omani community, with the rate estimated to be 56%,S32 owing to social, cultural, geographic, and economic factors.S30
In terms of molecular testing, the PKD1 gene is considered to be complex due to its large size (46 exons), the presence of 6 PKD1 pseudogenes, and its high allelic heterogeneity. Nonetheless, recent studies using capture-based methodology had successively covered all exonic regions of this gene.S33 Our results confirm that we were capable of sequencing, using long-range polymerase chain reaction, all exons of the PKD1 gene and detecting different types of genomic variants, thus providing accurate genotyping data of ADPKD patients. Eight pathogenic variants of PKD1 were identified in 10 suspected ADPKD patients (from 8 different families). We failed to solve 4 cases in which the clinical diagnosis of ADPKD was suspected. The coverage of PKD1 exons 1, 12, and 42 (Supplementary Table S4) was lower than the remaining exons but acceptable. These cases now need to be examined for copy number and structural variants in PKD1 as well as alternative genetic causes, including DNAJB11 S10 and GANAB,S9 which were not included on our panel.
ARPKD is a severe, early-onset type of cystic kidney disease caused by biallelic mutations in the PKHD1 gene, which consists of 67 exons that encode a 4074 amino acid type I transmembrane protein called fibrocystin/polyductin.S3 Almost 748 PKHD1 variants have been reported to date in the ARPKD mutation database (www.humgen.rwth-aachen.de), scattered along the entire length of the gene with no mutational hot spots.S34 Missense variation is a common mechanism of disease in the PKHD1 gene, where half of the reported variants are missense. Most of the PKHD1 mutations are private, and most PKHD1 patients may have compound heterozygous mutations. ARPKD is one of the most common genetic disorders in Oman, with an estimated birth incidence of 1 in 12,000 births.S29 In this study, only 4 different pathogenic missense variants were identified in PKHD1. The four variants, p.(Thr36Met), p.(Thr136Ala), p.(Arg1624Trp), and p.(His3124Tyr), involve substitutions of highly conserved amino acids and were all reported previously, except p.(Thr136Ala). The high frequency of these missense mutations suggests founder mutations in PKHD1 in Omani patients.
NPHP disease-causative variants were detected in 10 of 15 patients suspected clinically to have NPHP. Among Omani NPHP patients, NPHP1 gene deletion was identified in 3 unrelated patients from the same geographic region. NPHP3 mutations were responsible for infantile and juvenile NPHP in consanguineous families, where a novel deletion c.2529delA was associated with neonatal death and novel nonsense associated with cystic kidneys, hepatic fibrosis, and splenomegaly leading to ESRD before 1 year of age.
The lack of identification of causative variants in 13 (25%) patients, including 5 with suspected NPHP and 4 with multisystem ciliopathies, supports the genetic heterogeneity of renal ciliopathies, for which the spectrum of associated genes is continually expanding and the gene panel failed to include the latest known causes. For such patients, NGS panels containing additional known NPHP and renal ciliopathy genes, or whole-exome sequencing/whole-genome sequencing approaches, need to be applied. In addition, deletion-duplication analysis using multiplex ligation-dependent probe amplification in candidate genes and chromosomal microarray analysis are other future approaches for our unsolved cases. There is a growing argument to move to whole-exome sequencing and whole-genome sequencing approaches for molecular genetic diagnostics, given the constant need to update gene panels, the ability of whole-genome sequencing to provide information on structural variant and gene copy number, and the falling costs of these unselected methods. However, data analysis and interpretation continue to be challenges for such approaches and have slowed their implementation into molecular genetic diagnostic services.
In conclusion, we have demonstrated that for inherited cystic kidney disease, a targeted NGS panel is a comprehensive, noninvasive, and efficient tool for genetic diagnosis of patients. With this approach, a diagnostic yield of 75% was obtained in Omani patients with inherited cystic kidney disease. In addition, NGS panel sequencing allows large disease genes, such as PKD1, to be sequenced. This study represents a comprehensive molecular genetic overview of Omani patients with inherited cystic kidney disease and their associated clinical phenotypes and contributes to the knowledge of causative mutations in renal ciliopathy genes.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank the staff at the National Genetic Centre, Muscat, Oman, and the cooperative nephrologists and nurses from different referral hospitals throughout Oman for their kind collaboration. We also appreciate the cooperation of all members of the tested families. This study was supported by the Ministry of Health and The Research Council Grant (ORG/HSS/14/015), Oman, and the Northern Counties Kidney Research Fund, UK (awarded to JAS).
Footnotes
Supplementary Methods.
Supplementary References.
Figure S1. Geographic distribution of patients with cystic kidney disease included in this study.
Figure S2. Detection of NPHP1 deletion using target next-generation sequencing panel and confirmation by comparative genomic hybridization array.
Table S1. Disease categories and genes selected for next-generation sequencing panel for cystic kidney disease.
Table S2. Summary of design from cystic kidney disease next-generation sequencing panel.
Table S3. Next-generation sequencing panel performance metrics.
Table S4. Depth of coverage of the captured regions of the PKD1 gene using next-generation sequencing panel.
Table S5. Clinical phenotype and evidence of family history of renal disease.
Supplementary Material
References
- 1.Rossetti S., Hopp K., Sikkink R.A. Identification of gene mutations in autosomal dominant polycystic kidney disease through targeted resequencing. J Am Soc Nephrol. 2012;23:915–933. doi: 10.1681/ASN.2011101032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halbritter J., Porath J.D., Diaz K.A. Identification of 99 novel mutations in a worldwide cohort of 1,056 patients with a nephronophthisis-related ciliopathy. Hum Genet. 2013;132:865–884. doi: 10.1007/s00439-013-1297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onuchic L.F., Furu L., Nagasawa Y. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet. 2002;70:1305–1317. doi: 10.1086/340448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furu L., Onuchic L.F., Gharavi A. Milder presentation of recessive polycystic kidney disease requires presence of amino acid substitution mutations. J Am Soc Nephrol. 2003;14:2004–2014. doi: 10.1097/01.asn.0000078805.87038.05. [DOI] [PubMed] [Google Scholar]
- 5.Ward C.J., Hogan M.C., Rossetti S. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30:259–269. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 6.Otto E.A., Hurd T.W., Airik R. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat Genet. 2010;42:840–850. doi: 10.1038/ng.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edghill E.L., Bingham C., Ellard S. Mutations in hepatocyte nuclear factor-1beta and their related phenotypes. J Med Genet. 2006;43:84–90. doi: 10.1136/jmg.2005.032854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hildebrandt F., Otto E., Rensing C. A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat Genet. 1997;17:149–153. doi: 10.1038/ng1097-149. [DOI] [PubMed] [Google Scholar]
- 9.Bellanne-Chantelot C., Chauveau D., Gautier J.F. Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann Intern Med. 2004;140:510–517. doi: 10.7326/0003-4819-140-7-200404060-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.