Abstract
In this study, we have synthesized activated tungstic acid by dehydration of tungstic acid as a highly efficient catalyst and employed it for the catalytic transformations of various alkenes. The importance of this series of studies is to help students to graphically visualize a diverse array of organic synthesis reactions. The alkenes react differently depending on their structure via mainly acid-catalyzed reactions that produce a whole range of products including coupled products (dimers). For example, Cyclopentene was transformed into 1,1′-bi (cyclopent-3-ene) in yield 100% at room temperature. Cyclohexene-3-cyclohexyl was form from Cyclohexene. The synthesized product was identified and confirmed by gas chromatography and mass spectroscopy, measuring the retention times of authentic samples of the compounds.
Keywords: Organic chemistry, Synthesis, Catalyst, Cyclohexene, Cyclopentene, Tungstic acid
Organic chemistry; Synthesis; Catalyst; Cyclohexene; Cyclopentene; Tungstic acid
1. Introduction
Tungsten was identified by the Spanish brothers Juan José and Fausto de Elhuyar in 1783. The yellow oxides of this metal had been well known by scientists of that time as it occurs in the form of hydrated species, WO3.nH2O (n = 1,2), which form when tungsten-containing solutions are acidified. The monohydrate, WO3.H2O, is the material known as tungstic acid. Examples of these are the dark blue W20O58 (WO2.9), violet W18O49 (WO2.72), and chocolate brown WO2 and WO3. The color variations are due to the loss of oxygen that generates additional valence states (W5+ or W4+) in the WO3 parent structure. Cation-to-cation charge (also called intervalence charge transfer) between the parent W6+ and a reduced ion is accountable for the change in color [1].
A new method was developed for the production of an activated tungstic acid by dehydration of tungstic acid. This material is a significantly more active catalyst than the parent tungstic acid and often reacts with substrates at room temperature. This experiment aims to prepare 3-Cyclohexylcyclohexene from Cyclohexene, 1-methyl cyclohexanol from 1-methyl-1-cyclohexene, Cyclohexane 1,1′-oxybis from Cyclohexanol, and cyclopentene was transformed into 1, 1′-bi (cyclopent-3-ene) at 100% yield at room temperature using tungstic acid as the catalysis. The experiment begins by a dimer reaction leading to the formation of an oligomer structure consisting of similar monomers joined by intermolecular bonds forming Cyclohexene, 3-cyclohexyl. Cyclohexene may react in the presence of various catalysts like acids, initiators with reducing agents that exothermically release hydrogen gas, and strong oxidizing agents. The second reaction is hydration of 1-methyl-1-cyclohexene to form an alcohol named cyclohexanol 1-methyl. Heating converts cyclohexanol into Cyclohexene in the presence of acid catalysts [2]. Here, hydration is carried out for 1-methylcyclohexene conversion to 1-methylcyclohexanol. In recent years for cyclohexanol production, a liquid phase dehydration process has been widely used. One study described the preparation of cyclohexanol in the presence of a solid super acid SO42/TiO2–SiO2 catalyst. The catalyst amount was 10w% of cyclohexyl alcohol, the temperature was 170 °C, dehydration occurred for 1 h, and the yield was 90% with high purity [3]. The third reaction is the dehydration of alcohol in the presence of a tungstic acid as a catalyst to form ether. As reported cyclohexanone (95%) at elevated conversion of phenol (85%) can be achieved by conversion (99.9%) and selectivity (99.9%) by using an AlCl3–Pd/C dual catalyst [4]. The dehydration of alcohols produce ethers as 2R–OH →R–O–R + H2O when subjected to high temperature. Acid-catalyzed dehydration of alcohols is a special method that results in symmetrical ethers [5, 6].
The synthesized compounds were identified by comparing their mass spectra to the National Institute of Standards and Technology (NIST) database. In the identification of the target molecule, this spectrum serves as a fingerprint. The obtained spectrum should be compared with a verified reference spectrum from a database, namely, the NIST/EPA/NIH Mass Spectral Library. Product identity was confirmed by measuring the retention times of authentic samples of the compounds identified by mass spectroscopy. Analysis by gas chromatography/mass spectroscopy (GC-MS) serves both as a qualitative and quantitative analytical tool [7, 8]. The objectives of this experiment are to isolate 3-Cyclohexylcyclohexene from Cyclohexene, cyclohexanol 1-methyl from 1-methyl-1-cyclohexene, and Cyclohexane 1,1′-oxybis from Cyclohexanol by using tungstic acid as the catalysis and to determine the identity of the distillate product through gas chromatography/mass spectroscopy (GC-MS). The resultant solution analyzed with GC/MS is checked for purity and impurities intact with the compound produced.
2. Experimental
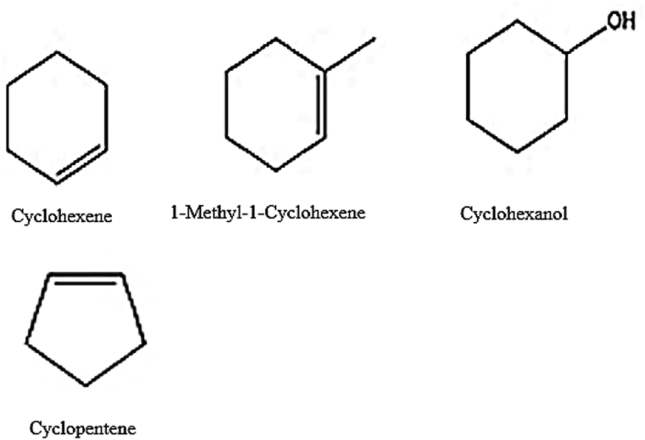
All organic reagents were commercial products and were used without purification (see Fig. 1).
Fig. 1.
Alkenes and alcohols that were used to investigate the catalytic activity for tungstic acid.
2.1. Synthesis of tungstic acid
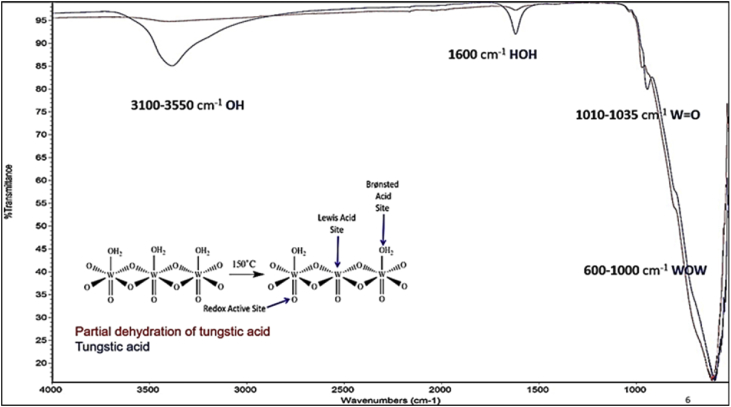
Activated tungstic acid was prepared by dehydration of tungstic acid at 150°C for 24 h (Fig. 2). Dehydration of tungstic acid in oven then protected in desiccators. Reactions were performed in glass culture tubes using an excess of the activated tungstic acid (2.5 g) and 0.25 g of the alkenes. The sealed reactors were placed in an oven at various temperatures under autogenous pressure. The amounts of reactants and products in the reaction mixtures were determined by cooling the reaction, then the products were extracted by different solvents (such as CH2Cl2, and Pentene). After the extraction, the sample was analyzed by chromatography/mass spectrometry. Compounds were identified by comparison of their mass spectra to the NIST database. The resulting identity was confirmed by measuring the retention times of authentic samples of the compounds identified by mass spectrometry.
Fig. 2.
Shows a spectrum of IR for partial dehydrogenation of tungstic acid at 150°C for day and tungstic acid.
3. Results and discussion
3.1. Synthesis of cyclopentene
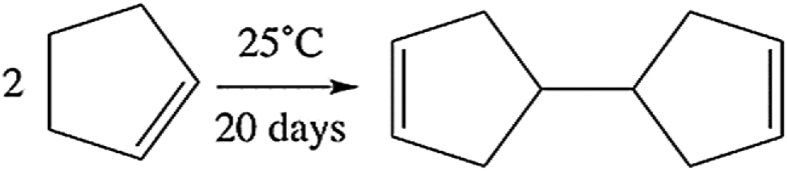
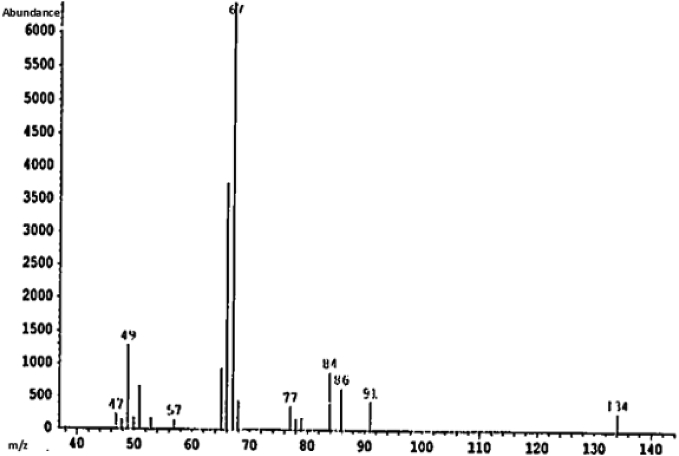
The smallest and simplest substrate to be tested was cyclopentene. Activated tungstic acid was added to cyclopentene, and the reaction mixture was stirred for 20 days at room temperature. During the course of the reaction, the tungstic acid changed from yellow to green. The reaction cleanly produced the dimeric product shown in (Fig. 3) that was identified by GC/MS (Fig. 4) and was isolated in a quantitative yield.
Fig. 3.
The reaction cleanly produced the dimeric product in a quantitative yield.
Fig. 4.
Mass spectrum of the Cyclopentene reaction product.
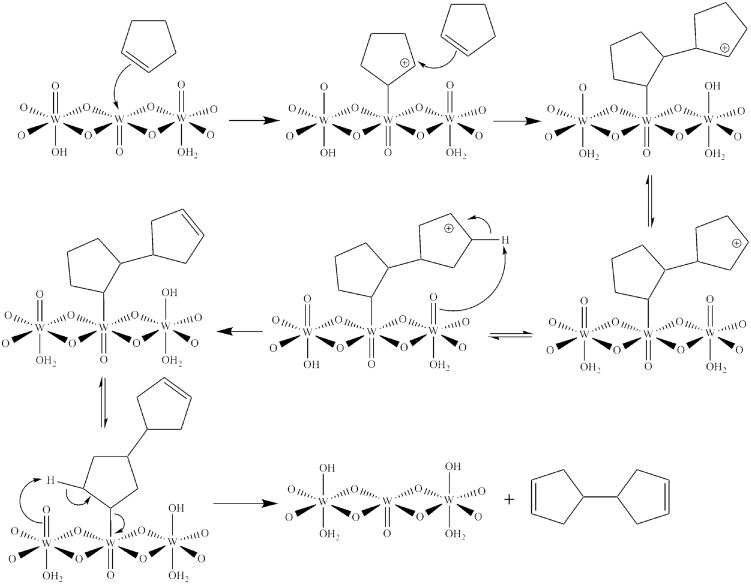
The product from the reaction was unexpected since 4-cyclopentyl cyclopentene was the predicted product from acid-catalyzed dimerization of cyclopentene. For this reason, it is proposed that the reaction with tungstic acid involves the Lewis acid sites. In this mechanism (as shown in Scheme 1), the double bond of a cyclopentene coordinates to a Lewis acidic tungsten center, generating a carbocation that is attacked by the double bond of a second cyclopentene. The new carbocation rearranges by a series of hydride shifts that move a hydrogen atom to a point where it can react with a tungsten oxo group. At this point, a redox reaction occurs, producing a W(V) center (W–OH) and an alkene. The formation of W(V) is the cause of the resulting green color (Fig. 5). A similar series of reactions of the bound cyclopentene (shift of the W bond followed by a redox reaction) occurs to release the 1,1′-dicyclo (3-pentene) (see Fig. 6).
Scheme 1.
Proposed mechanism for formation of 1,1′-dicyclo (3-pentene).
Fig. 5.
Color change of tungstic acid reaction with Cyclopentene for 20 days.
Fig. 6.
13C NMR spectrum for the Cyclopentene product.
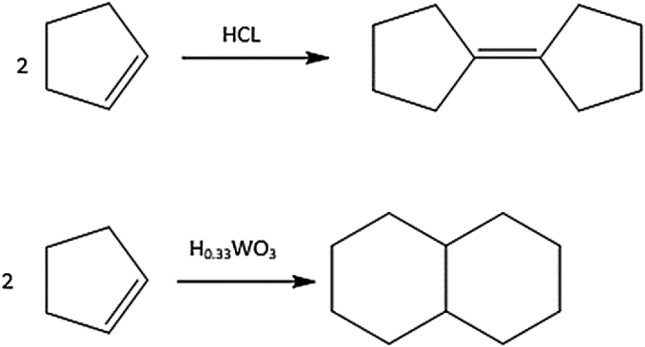
This product is quite different from the dimeric products produced by other catalysts, as shown in (Fig. 7) [9]. Hydrochloric acid yields a product where the originally formed 4-cyclopentyl cyclopentene rearranges to move the double bond between two rings [9]. On the other hand, tungsten hydrogen bronze and Lewis acid convert cyclopentene into decalin. Notably, the transformation of two cyclopentene molecules into two fused six-membered rings is an important reaction for the discussion of the indene/activated tungstic acid reactions. It is somewhat surprising that the dialkene product does not react further, but this may be partly due to the low temperature. Notably, the product, as a diene, is expected to be a useful cross-linker for polymers (see Figs. 8 and 9).
Fig. 7.
Cyclopentene reaction with different catalyses.
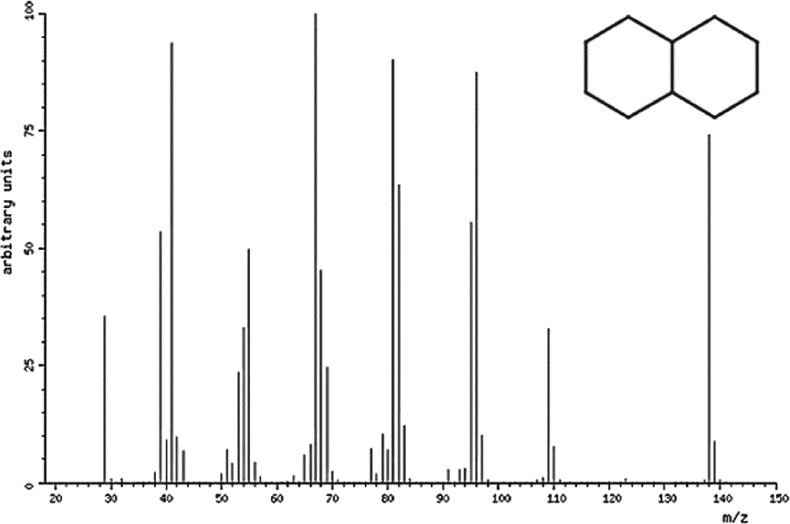
Fig. 8.
Mass spectrum of the Naphthalene, decahydro- [10].
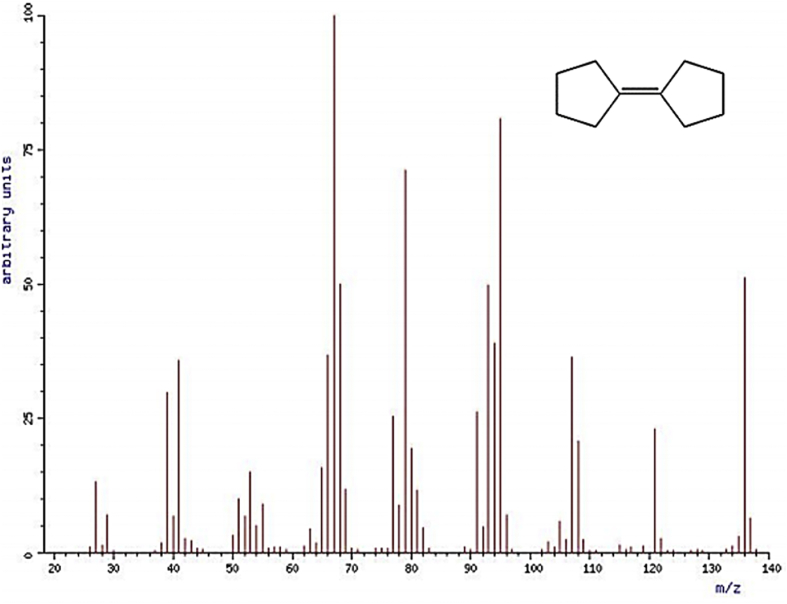
Fig. 9.
Mass spectrum of the Cyclopentane, cyclopentylidene- [10].
3.2. Cyclohexene and 1-methylcyclohexene
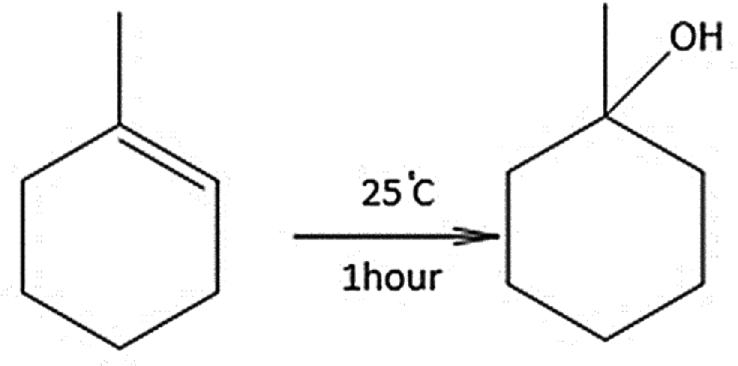
Cyclohexene normally reacts with strong acids to produce cyclohexanol, an important intermediate in industrial chemistry. In this investigation, it was found that activated tungstic acid produced 1-cyclohexyl cyclohexene (Fig. 10) at 25 °C. The reaction is complete within an hour, and the product was identified by GC/MS (Fig. 11).
Fig. 10.

Formation of 3-Cyclohexylhexene from cyclohexene.
Fig. 11.
Mass spectra of the 3-Cyclohexylcyclohexene product.
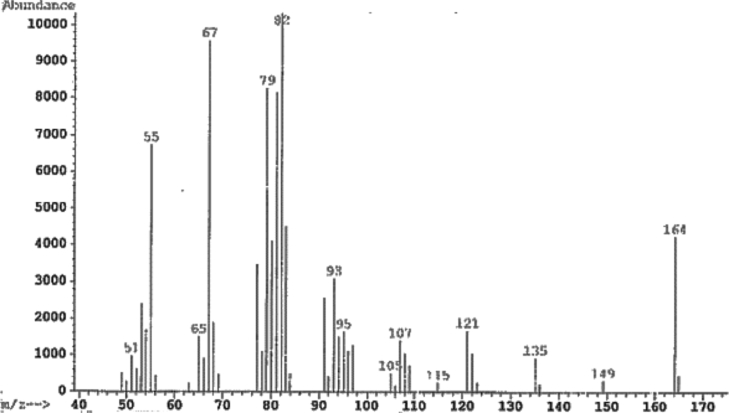
Under the same conditions as cyclohexene, 1-Methycyclohexene gave a quantitative yield of 1-methyl cyclohexanol (Fig. 12). This is the expected hydration product formed according to Markovnikov's rule. It is likely that hydration occurs due to steric hindrance of the methyl group blocking the attack of a second alkene. The mass spectrum of the product and its match to the NIST database are shown in (Fig. 13) [11, 12].
Fig. 12.
Formation of 1-methylcyclohexanol from 1-methyl-1-cyclohexene.
Fig. 13.
Mass spectra of 1-Methylcyclohexanol from 1-Methyl-1-cyclohexene.
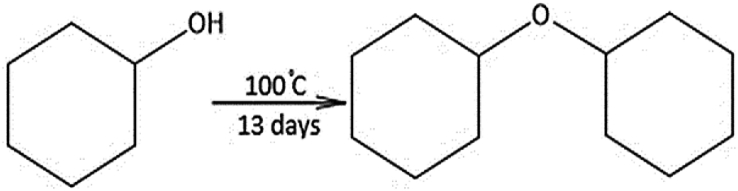
Cyclohexanol was heated at 100°C with tungstic acid and was found to be converted to dicyclohexyl ether through a condensation reaction between two alcohol molecules that released water as a byproduct. After 13 days, complete conversion of the cyclohexanol to the ether products was observed (Fig. 14). The mass spectrum of the product is shown in (Fig. 15).
Fig. 14.
Formation of Dicyclohexyl ether from Cyclohexanol.
Fig. 15.
Mass spectra of the Cyclohexanol product.
4. Conclusions
Activated tungstic acid is an environmentally friendly solid catalyst that is capable of catalyzing alkene reactions at room temperature. It can be easily recovered and reused. These reactions gave a wide range of products depending on the substrate and the reaction temperature used. In several cases, unique products were obtained. It was observed in this experiment that the mass spectrum of the product obtained is relatively close to the theoretical molecular weight of the original compounds. The synthesis of 3-Cyclohexylcyclohexene from Cyclohexene, cyclohexanol 1-methyl from 1-methyl-1-cyclohexene, and Cyclohexane 1,1′-oxybis from Cyclohexanol was able to perform all of the objectives for this experiment, which included synthesizing products from a precursor compound and obtaining a mass spectrum of the product via gas chromatography-mass spectrometry. The synthesis of products can be done in many ways, and the one used in the present study is one of its examples. The products obtained were 100% Cyclohexene 3-cyclohexyl, 100% cyclohexanol 1-methyl, and 54% Cyclohexane 1,1′-oxybis with other impurities such as methyl Cyclopentanol, Cyclohexyl-Cyclohexene isomers, and dicyclohexyl ether in cyclohexanol.
Declarations
Author contribution statement
Allen W. Apblett: Conceived and designed the experiments.
Salhah Hamed Alrefaee: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Tilley R.J.D. Wiley; Hoboken, NJ: 2011. Colour and the Optical Properties of Materials: an Exploration of the Relationship between Light, the Optical Properties of Materials and Colour. [Google Scholar]
- 2.Tuttle Musser Michael. Wiley-VCH; Weinheim: 2005. Cyclohexanol and Cyclohexanone" in Ullmann's Encyclopedia of Industrial Chemistry. [Google Scholar]
- 3.Junsheng G. Preparation of cyclohexene by catalyzed dehydration of cyclohexanol. Chem. Reagents. 2001;23(3):178–179. [Google Scholar]
- 4.Liu H., Jiang T., Han B., Liang S., Zhou Y. Selective phenol hydrogenation to cyclohexanone over a dual supported Pd–Lewis acid catalyst. Science. 2009;326(5957):1250–1252. doi: 10.1126/science.1179713. [DOI] [PubMed] [Google Scholar]
- 5.Zhou L., Xu J., Miao H., Wang F., Li X. Catalytic oxidation of cyclohexane to cyclohexanol and cyclohexanone over Co 3 O 4 nanocrystals with molecular oxygen. Appl. Catal. Gen. 2005;292:223–228. [Google Scholar]
- 6.Jeon G.S., Chung J.S. Preparation and characterization of silica-supported copper catalysts for the dehydrogenation of cyclohexanol to cyclohexanone. Appl. Catal. Gen. 2004;115(1):29–44. [Google Scholar]
- 7.Fernie A.R., Trthewey R.N., Krotzky A.J., Willmitzer L. Metabolite profiling: from diagnostics to systems biology. Nat. Rev. 2004;5:1–7. doi: 10.1038/nrm1451. [DOI] [PubMed] [Google Scholar]
- 8.Fiehn O., Kopka J., Trethewey R.N., Willmitzer L. Identification of uncommonplant metabolites based on calculation of elemental compositions using gas chromatography and quadrupole mass spectrometry. Anal. Chem. 2000;72:3575–3580. doi: 10.1021/ac991142i. [DOI] [PubMed] [Google Scholar]
- 9.Apblett A.W., Kiran B.P., Oden K. 2003. ACS Symposium Series,837, (Chlorinated Solvent and DNAPL Remediation; p. 154. [Google Scholar]
- 10.https://www.sisweb.com/software/ms/wiley.htm
- 11.Yarwood J., Douthwaite R., Duckett S., Ball G.E., Keyes T., Christensen P., Madejova J., Bakker H.J., Smith W.E., Slatterly J.M., Tuttle T., Eisenstein O. 2010. Spectroscopic Properties of Inorganic and Organometallic Compounds: Techniques, Materials and Applications. [Google Scholar]
- 12.Yarwood J., Douthwaite R., Duckett S. Royal Society of Chemistry; Cambridge: 2014. Spectroscopic Properties of Inorganic and Organometallic Compounds: Techniques, Materials and Applications. [Google Scholar]